[English] 日本語
 Yorodumi
Yorodumi- EMDB-42837: Cryo-EM structure of GeoCas9 in complex with sgRNA and target DNA -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of GeoCas9 in complex with sgRNA and target DNA | |||||||||
 Map data Map data | Sharp map of deactivated WT-GeoCas9 with sgRNA and target DNA. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA-guided DNA endonuclease / ternary complex / CRISPR / hydrolase / HYDROLASE-RNA-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationmaintenance of CRISPR repeat elements / endonuclease activity / defense response to virus / Hydrolases; Acting on ester bonds / hydrolase activity / DNA binding / RNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |   Geobacillus stearothermophilus (bacteria) / Geobacillus stearothermophilus (bacteria) /  | |||||||||
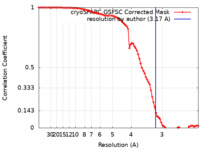
| Method | single particle reconstruction / cryo EM / Resolution: 3.17 Å | |||||||||
 Authors Authors | Eggers AR / Soczek KM / Tuck OT / Doudna JA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2024 Journal: Cell / Year: 2024Title: Rapid DNA unwinding accelerates genome editing by engineered CRISPR-Cas9. Authors: Amy R Eggers / Kai Chen / Katarzyna M Soczek / Owen T Tuck / Erin E Doherty / Bryant Xu / Marena I Trinidad / Brittney W Thornton / Peter H Yoon / Jennifer A Doudna /  Abstract: Thermostable clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (Cas9) enzymes could improve genome-editing efficiency and delivery due to extended protein ...Thermostable clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (Cas9) enzymes could improve genome-editing efficiency and delivery due to extended protein lifetimes. However, initial experimentation demonstrated Geobacillus stearothermophilus Cas9 (GeoCas9) to be virtually inactive when used in cultured human cells. Laboratory-evolved variants of GeoCas9 overcome this natural limitation by acquiring mutations in the wedge (WED) domain that produce >100-fold-higher genome-editing levels. Cryoelectron microscopy (cryo-EM) structures of the wild-type and improved GeoCas9 (iGeoCas9) enzymes reveal extended contacts between the WED domain of iGeoCas9 and DNA substrates. Biochemical analysis shows that iGeoCas9 accelerates DNA unwinding to capture substrates under the magnesium-restricted conditions typical of mammalian but not bacterial cells. These findings enabled rational engineering of other Cas9 orthologs to enhance genome-editing levels, pointing to a general strategy for editing enzyme improvement. Together, these results uncover a new role for the Cas9 WED domain in DNA unwinding and demonstrate how accelerated target unwinding dramatically improves Cas9-induced genome-editing activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42837.map.gz emd_42837.map.gz | 60.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42837-v30.xml emd-42837-v30.xml emd-42837.xml emd-42837.xml | 23.4 KB 23.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42837_fsc.xml emd_42837_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_42837.png emd_42837.png | 98.5 KB | ||
| Masks |  emd_42837_msk_1.map emd_42837_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-42837.cif.gz emd-42837.cif.gz | 7.4 KB | ||
| Others |  emd_42837_additional_1.map.gz emd_42837_additional_1.map.gz emd_42837_half_map_1.map.gz emd_42837_half_map_1.map.gz emd_42837_half_map_2.map.gz emd_42837_half_map_2.map.gz | 49 MB 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42837 http://ftp.pdbj.org/pub/emdb/structures/EMD-42837 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42837 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42837 | HTTPS FTP |
-Related structure data
| Related structure data |  8uzaMC  8uzbC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42837.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42837.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharp map of deactivated WT-GeoCas9 with sgRNA and target DNA. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.93 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_42837_msk_1.map emd_42837_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Map of deactivated WT-GeoCas9 with sgRNA and target DNA.
| File | emd_42837_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map of deactivated WT-GeoCas9 with sgRNA and target DNA. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of deactivated WT-GeoCas9 with sgRNA and target DNA.
| File | emd_42837_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of deactivated WT-GeoCas9 with sgRNA and target DNA. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of deactivated WT-GeoCas9 with sgRNA and target DNA.
| File | emd_42837_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of deactivated WT-GeoCas9 with sgRNA and target DNA. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of deactivated wild type GeoCas9 with sgRNA and t...
| Entire | Name: Ternary complex of deactivated wild type GeoCas9 with sgRNA and target DNA |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of deactivated wild type GeoCas9 with sgRNA and t...
| Supramolecule | Name: Ternary complex of deactivated wild type GeoCas9 with sgRNA and target DNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Geobacillus stearothermophilus (bacteria) Geobacillus stearothermophilus (bacteria) |
| Molecular weight | Theoretical: 203 KDa |
-Macromolecule #1: CRISPR-associated endonuclease Cas9
| Macromolecule | Name: CRISPR-associated endonuclease Cas9 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Geobacillus stearothermophilus (bacteria) Geobacillus stearothermophilus (bacteria) |
| Molecular weight | Theoretical: 127.043883 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRYKIGLAIG ITSVGWAVMN LDIPRIEDLG VRIFDRAENP QTGESLALPR RLARSARRRL RRRKHRLERI RRLVIREGIL TKEELDKLF EEKHEIDVWQ LRVEALDRKL NNDELARVLL HLAKRRGFKS NRKSERSNKE NSTMLKHIEE NRAILSSYRT V GEMIVKDP ...String: MRYKIGLAIG ITSVGWAVMN LDIPRIEDLG VRIFDRAENP QTGESLALPR RLARSARRRL RRRKHRLERI RRLVIREGIL TKEELDKLF EEKHEIDVWQ LRVEALDRKL NNDELARVLL HLAKRRGFKS NRKSERSNKE NSTMLKHIEE NRAILSSYRT V GEMIVKDP KFALHKRNKG ENYTNTIARD DLEREIRLIF SKQREFGNMS CTEEFENEYI TIWASQRPVA SKDDIEKKVG FC TFEPKEK RAPKATYTFQ SFIAWEHINK LRLISPSGAR GLTDEERRLL YEQAFQKNKI TYHDIRTLLH LPDDTYFKGI VYD RGESRK QNENIRFLEL DAYHQIRKAV DKVYGKGKSS SFLPIDFDTF GYALTLFKDD ADIHSYLRNE YEQNGKRMPN LANK VYDNE LIEELLNLSF TKFGHLSLKA LRSILPYMEQ GEVYSSACER AGYTFTGPKK KQKTMLLPNI PPIANPVVMR ALTQA RKVV NAIIKKYGSP VSIHIELARD LSQTFDERRK TKKEQDENRK KNETAIRQLM EYGLTLNPTG HDIVKFKLWS EQNGRC AYS LQPIEIERLL EPGYVEVDAV IPYSRSLDDS YTNKVLVLTR ENREKGNRIP AEYLGVGTER WQQFETFVLT NKQFSKK KR DRLLRLHYDE NEETEFKNRN LNDTRYISRF FANFIREHLK FAESDDKQKV YTVNGRVTAH LRSRWEFNKN REESDLHH A VDAVIVACTT PSDIAKVTAF YQRREQNKEL AKKTEPHFPQ PWPHFADELR ARLSKHPKES IKALNLGNYD DQKLESLQP VFVSRMPKRS VTGAAHQETL RRYVGIDERS GKIQTVVKTK LSEIKLDASG HFPMYGKESD PRTYEAIRQR LLEHNNDPKK AFQEPLYKP KKNGEPGPVI RTVKIIDTKN QVIPLNDGKT VAYNSNIVRV DVFEKDGKYY CVPVYTMDIM KGILPNKAIE P NKPYSEWK EMTEDYTFRF SLYPNDLIRI ELPREKTVKT AAGEEINVKD VFVYYKTIDS ANGGLELISH DHRFSLRGVG SR TLKRFEK YQVDVLGNIY KVRGEKRVGL ASSAHSKPGK TIRPLQSTRD UniProtKB: CRISPR-associated endonuclease Cas9 |
-Macromolecule #2: sgRNA (107-MER)
| Macromolecule | Name: sgRNA (107-MER) / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:   Geobacillus stearothermophilus (bacteria) Geobacillus stearothermophilus (bacteria) |
| Molecular weight | Theoretical: 44.488117 KDa |
| Sequence | String: CACUGCAUUC UAGUUGUGGU UGUCAUAGUU CCCCUGAGAA AUCAGGGUUA CUAUGAUAAG GGCUUUCUGC CUAAGGCAGA CUGACCCGC GGCGUUGGGG AUCGCCUGUC GCCCGCUUUU GGCGGGCAUU CCCCAUCCUU |
-Macromolecule #3: Target strand DNA
| Macromolecule | Name: Target strand DNA / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.797221 KDa |
| Sequence | String: (DT)(DA)(DC)(DA)(DT)(DT)(DG)(DA)(DT)(DG) (DA)(DG)(DT)(DT)(DT)(DG)(DG)(DA)(DC)(DA) (DA)(DA)(DC)(DC)(DA)(DC)(DA)(DA)(DC) (DT)(DA)(DG)(DA)(DA)(DT)(DG)(DC)(DA)(DG) (DT) (DG)(DA)(DA)(DA)(DA)(DA)(DA)(DA) (DT)(DG)(DC) |
-Macromolecule #4: Non-target strand DNA
| Macromolecule | Name: Non-target strand DNA / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.618021 KDa |
| Sequence | String: (DG)(DC)(DA)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DC)(DA)(DC)(DT)(DG)(DC)(DA)(DT)(DT)(DC) (DT)(DA)(DG)(DT)(DT)(DG)(DT)(DG)(DG) (DT)(DT)(DT)(DG)(DT)(DC)(DC)(DA)(DA)(DA) (DC) (DT)(DC)(DA)(DT)(DC)(DA)(DA)(DT) (DG)(DT)(DA) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: OTHER | ||||||
| Output model |  PDB-8uza: |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)