[English] 日本語
 Yorodumi
Yorodumi- EMDB-42804: Structure of nucleotide-free Pediculus humanus (Ph) PINK1 dimer -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of nucleotide-free Pediculus humanus (Ph) PINK1 dimer | |||||||||
 Map data Map data | Map (unsharpened) of the nucleotide-free PhPINK1 dimer | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | PINK1 / Kinase / Mitophagy / Parkinson's Disease / Ubiquitin / Phosphorylation / Phospho-ubiquitin / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationautophagy of mitochondrion / positive regulation of mitochondrial fission / regulation of apoptotic process / mitochondrial outer membrane / protein kinase activity / non-specific serine/threonine protein kinase / mitochondrial inner membrane / protein serine/threonine kinase activity / ATP binding / metal ion binding / cytosol Similarity search - Function | |||||||||
| Biological species |  Pediculus humanus corporis (human body louse) Pediculus humanus corporis (human body louse) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.75 Å | |||||||||
 Authors Authors | Gan ZY / Kirk NS / Leis A / Komander D | |||||||||
| Funding support |  Australia, Australia,  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2024 Journal: Sci Adv / Year: 2024Title: Interaction of PINK1 with nucleotides and kinetin. Authors: Zhong Yan Gan / Sylvie Callegari / Thanh N Nguyen / Nicholas S Kirk / Andrew Leis / Michael Lazarou / Grant Dewson / David Komander /  Abstract: The ubiquitin kinase PINK1 accumulates on damaged mitochondria to trigger mitophagy, and PINK1 loss-of-function mutations cause early onset Parkinson's disease. Nucleotide analogs such as kinetin ...The ubiquitin kinase PINK1 accumulates on damaged mitochondria to trigger mitophagy, and PINK1 loss-of-function mutations cause early onset Parkinson's disease. Nucleotide analogs such as kinetin triphosphate (KTP) were reported to enhance PINK1 activity and may represent a therapeutic strategy for the treatment of Parkinson's disease. Here, we investigate the interaction of PINK1 with nucleotides, including KTP. We establish a cryo-EM platform exploiting the dodecamer assembly of () PINK1 and determine PINK1 structures bound to AMP-PNP and ADP, revealing conformational changes in the kinase N-lobe that help establish PINK1's ubiquitin binding site. Notably, we find that KTP is unable to bind PINK1 or human () PINK1 due to a steric clash with the kinase "gatekeeper" methionine residue, and mutation to Ala or Gly is required for PINK1 to bind and use KTP as a phosphate donor in ubiquitin phosphorylation and mitophagy. PINK1 M318G can be used to conditionally uncouple PINK1 stabilization and activity on mitochondria. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42804.map.gz emd_42804.map.gz | 31.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42804-v30.xml emd-42804-v30.xml emd-42804.xml emd-42804.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42804_fsc.xml emd_42804_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_42804.png emd_42804.png | 74.3 KB | ||
| Filedesc metadata |  emd-42804.cif.gz emd-42804.cif.gz | 6.1 KB | ||
| Others |  emd_42804_half_map_1.map.gz emd_42804_half_map_1.map.gz emd_42804_half_map_2.map.gz emd_42804_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42804 http://ftp.pdbj.org/pub/emdb/structures/EMD-42804 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42804 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42804 | HTTPS FTP |
-Related structure data
| Related structure data |  8uyfMC  8uyhC  8uyiC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_42804.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42804.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map (unsharpened) of the nucleotide-free PhPINK1 dimer | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.808 Å | ||||||||||||||||||||||||||||||||||||
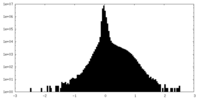
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map of the nucleotide-free PhPINK1 dimer
| File | emd_42804_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of the nucleotide-free PhPINK1 dimer | ||||||||||||
| Projections & Slices |
| ||||||||||||
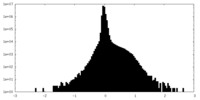
| Density Histograms |
-Half map: Half map of the nucleotide-free PhPINK1 dimer
| File | emd_42804_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of the nucleotide-free PhPINK1 dimer | ||||||||||||
| Projections & Slices |
| ||||||||||||
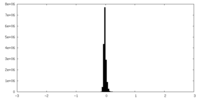
| Density Histograms |
- Sample components
Sample components
-Entire : Nucleotide-free Pediculus humanus (Ph) PINK1 dodecamer
| Entire | Name: Nucleotide-free Pediculus humanus (Ph) PINK1 dodecamer |
|---|---|
| Components |
|
-Supramolecule #1: Nucleotide-free Pediculus humanus (Ph) PINK1 dodecamer
| Supramolecule | Name: Nucleotide-free Pediculus humanus (Ph) PINK1 dodecamer type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Pediculus humanus corporis (human body louse) Pediculus humanus corporis (human body louse) |
-Macromolecule #1: Serine/threonine-protein kinase Pink1, mitochondrial
| Macromolecule | Name: Serine/threonine-protein kinase Pink1, mitochondrial / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pediculus humanus corporis (human body louse) Pediculus humanus corporis (human body louse) |
| Molecular weight | Theoretical: 53.053602 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPSGLLTKDD ELEGICWEIR EAVSKGKWND SESENVEQLQ AANLDELDLG EPIAKGCNAV VYSAKLKNVQ SNKLAHQLAV KMMFNYDVE SNSTAILKAM YRETVPAMSY FFNQNLFNIE NISDFKIRLP PHPNIVRMYS VFADRIPDLQ CNKQLYPEAL P PRINPEGS ...String: GPSGLLTKDD ELEGICWEIR EAVSKGKWND SESENVEQLQ AANLDELDLG EPIAKGCNAV VYSAKLKNVQ SNKLAHQLAV KMMFNYDVE SNSTAILKAM YRETVPAMSY FFNQNLFNIE NISDFKIRLP PHPNIVRMYS VFADRIPDLQ CNKQLYPEAL P PRINPEGS GRNMSLFLVM KRYDCTLKEY LRDK(TPO)PNMRS SILLLSQLLE AVAHMNIHNI SHRDLKSDNI LVDLSEGD A YPTIVITDFG CCLCDKQNGL VIPYRSEDQD KGGNRALMAP EIANAKPGTF SWLNYKKSDL WAVGAIAYEI FNIDNPFYD KTMKLLSKSY KEEDLPELPD TIPFIIRNLV SNMLSRSTNK RLDCDVAATV AQLYLWAPSS WLKENYTLPN SNEIIQWLLC LSSKVLCER DITARNKTNT MSESVSKAQY KGRRSLPEYE LIASFLRRVR LHLVRKGLKW IQELHIYN UniProtKB: Serine/threonine-protein kinase Pink1, mitochondrial |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.9 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8.5 Component:
| ||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)