[English] 日本語
 Yorodumi
Yorodumi- EMDB-42281: Structure of the far-red light-absorbing allophycocyanin core exp... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the far-red light-absorbing allophycocyanin core expressed during FaRLiP | ||||||||||||
 Map data Map data | sharpened and masked | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | light-harvesting / allophycocyanin / phycocyanobilin / PHOTOSYNTHESIS | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationphotorespiration / carboxysome / ribulose-bisphosphate carboxylase / ribulose-bisphosphate carboxylase activity / reductive pentose-phosphate cycle / monooxygenase activity / magnesium ion binding Similarity search - Function | ||||||||||||
| Biological species |  Synechococcus sp. PCC 7335 (bacteria) Synechococcus sp. PCC 7335 (bacteria) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.35 Å | ||||||||||||
 Authors Authors | Gisriel CJ / Bryant DA / Brudvig GW / Shen G | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2024 Journal: J Biol Chem / Year: 2024Title: Structure of the antenna complex expressed during far-red light photoacclimation in Synechococcus sp. PCC 7335. Authors: Christopher J Gisriel / Gaozhong Shen / Gary W Brudvig / Donald A Bryant /  Abstract: Far-red light photoacclimation, or FaRLiP, is a facultative response exhibited by some cyanobacteria that allows them to absorb and utilize lower energy light (700-800 nm) than the wavelengths ...Far-red light photoacclimation, or FaRLiP, is a facultative response exhibited by some cyanobacteria that allows them to absorb and utilize lower energy light (700-800 nm) than the wavelengths typically used for oxygenic photosynthesis (400-700 nm). During this process, three essential components of the photosynthetic apparatus are altered: photosystem I, photosystem II, and the phycobilisome. In all three cases, at least some of the chromophores found in these pigment-protein complexes are replaced by chromophores that have red-shifted absorbance relative to the analogous complexes produced in visible light. Recent structural and spectroscopic studies have elucidated important features of the two photosystems when altered to absorb and utilize far-red light, but much less is understood about the modified phycobiliproteins made during FaRLiP. We used single-particle, cryo-EM to determine the molecular structure of a phycobiliprotein core complex comprising allophycocyanin variants that absorb far-red light during FaRLiP in the marine cyanobacterium Synechococcus sp. PCC 7335. The structure reveals the arrangement of the numerous red-shifted allophycocyanin variants and the probable locations of the chromophores that serve as the terminal emitters in this complex. It also suggests how energy is transferred to the photosystem II complexes produced during FaRLiP. The structure additionally allows comparisons with other previously studied allophycocyanins to gain insights into how phycocyanobilin chromophores can be tuned to absorb far-red light. These studies provide new insights into how far-red light is harvested and utilized during FaRLiP, a widespread cyanobacterial photoacclimation mechanism. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42281.map.gz emd_42281.map.gz | 9.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42281-v30.xml emd-42281-v30.xml emd-42281.xml emd-42281.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42281_fsc.xml emd_42281_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_42281.png emd_42281.png | 82.7 KB | ||
| Filedesc metadata |  emd-42281.cif.gz emd-42281.cif.gz | 5.7 KB | ||
| Others |  emd_42281_additional_1.map.gz emd_42281_additional_1.map.gz emd_42281_additional_2.map.gz emd_42281_additional_2.map.gz emd_42281_half_map_1.map.gz emd_42281_half_map_1.map.gz emd_42281_half_map_2.map.gz emd_42281_half_map_2.map.gz | 59.9 MB 45.5 MB 45.7 MB 45.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42281 http://ftp.pdbj.org/pub/emdb/structures/EMD-42281 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42281 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42281 | HTTPS FTP |
-Validation report
| Summary document |  emd_42281_validation.pdf.gz emd_42281_validation.pdf.gz | 935.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42281_full_validation.pdf.gz emd_42281_full_validation.pdf.gz | 935.5 KB | Display | |
| Data in XML |  emd_42281_validation.xml.gz emd_42281_validation.xml.gz | 16.1 KB | Display | |
| Data in CIF |  emd_42281_validation.cif.gz emd_42281_validation.cif.gz | 21 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42281 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42281 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42281 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42281 | HTTPS FTP |
-Related structure data
| Related structure data |  8uhiMC  8uheC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42281.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42281.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened and masked | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.825 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Far-red light-absorbing allophycocyanin core
| Entire | Name: Far-red light-absorbing allophycocyanin core |
|---|---|
| Components |
|
-Supramolecule #1: Far-red light-absorbing allophycocyanin core
| Supramolecule | Name: Far-red light-absorbing allophycocyanin core / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Synechococcus sp. PCC 7335 (bacteria) Synechococcus sp. PCC 7335 (bacteria) |
-Macromolecule #1: Ribulose bisphosphate carboxylase small subunit
| Macromolecule | Name: Ribulose bisphosphate carboxylase small subunit / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Synechococcus sp. PCC 7335 (bacteria) Synechococcus sp. PCC 7335 (bacteria) |
| Molecular weight | Theoretical: 13.517304 KDa |
| Sequence | String: MKTLPKERRY ETLSYLPPLT DQQIEKQINY CLQMGYIPAV EFNETSEPEA YYWTMWKLPL FGANNTRAVL DEIQACRSEY GNCFIRVVG FDNVKQCQAV SFIVHKPGSN NSSGYRY UniProtKB: Ribulose bisphosphate carboxylase small subunit |
-Macromolecule #2: Ribulose bisphosphate carboxylase large subunit
| Macromolecule | Name: Ribulose bisphosphate carboxylase large subunit / type: protein_or_peptide / ID: 2 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Synechococcus sp. PCC 7335 (bacteria) Synechococcus sp. PCC 7335 (bacteria) |
| Molecular weight | Theoretical: 53.031836 KDa |
| Sequence | String: MSYSQTQSKS GAGYDAGVQD YRLTYYAPDY TPRDTDILAA FRMTPQPGVP PEECAAAVAA ESSTGTWTTV WTDLLTDMDR YRGRCYDIE PVPGEDNQYI AYVAYPLDLF EEGSVTNLLT SLVGNVFGFK ALRALRLEDL RIPVAYVKTF QGPPHGIQVE R DRINKYGR ...String: MSYSQTQSKS GAGYDAGVQD YRLTYYAPDY TPRDTDILAA FRMTPQPGVP PEECAAAVAA ESSTGTWTTV WTDLLTDMDR YRGRCYDIE PVPGEDNQYI AYVAYPLDLF EEGSVTNLLT SLVGNVFGFK ALRALRLEDL RIPVAYVKTF QGPPHGIQVE R DRINKYGR PLLGCTIKPK LGLSAKNYGR AVYECLRGGL DFT(KCX)DDENIN SQPFQRWRDR FLFVADAIHK SQAETGEI K GHYLNVTAAT CEEMMKRAAY AKELEMPIVM HDFLTGGFTA NTTLAHWCRD NGILLHIHRA MHAVIDRQKN HGIHFRVLA KCLRMSGGDH IHTGTVVGKL EGDRAGTLGF VDLLRENYIE QDKSRGVYFT QDWASMPGVM AVASGGIHVW HMPALVEIFG DDSVLQFGG GTLGHPWGNA PGATANRVAL EACVQARNEG RNLAREGGDI IREACKWSPE LAAACELWKE IKFEFDTVDT I UniProtKB: Ribulose bisphosphate carboxylase large chain |
-Macromolecule #3: RIBULOSE-1,5-DIPHOSPHATE
| Macromolecule | Name: RIBULOSE-1,5-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 8 / Formula: RUB |
|---|---|
| Molecular weight | Theoretical: 310.09 Da |
| Chemical component information | 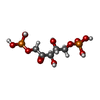 ChemComp-RUB: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 8 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 904 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER / Details: homology, see publication |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.35 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 57918 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller




