+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Consensus map of Sec7 dimer autoinhibited conformation | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GEF / Exchange Factor / Golgi / PROTEIN TRANSPORT | |||||||||
| Biological species |  Thermothielavioides terrestris (fungus) Thermothielavioides terrestris (fungus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.3 Å | |||||||||
 Authors Authors | Brownfield BA / Fromme JC | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: Sec7 regulatory domains scaffold autoinhibited and active conformations. Authors: Bryce A Brownfield / Brian C Richardson / Steve L Halaby / J Christopher Fromme /  Abstract: The late stages of Golgi maturation involve a series of sequential trafficking events in which cargo-laden vesicles are produced and targeted to multiple distinct subcellular destinations. Each of ...The late stages of Golgi maturation involve a series of sequential trafficking events in which cargo-laden vesicles are produced and targeted to multiple distinct subcellular destinations. Each of these vesicle biogenesis events requires activation of an Arf GTPase by the Sec7/BIG guanine nucleotide exchange factor (GEF). Sec7 localization and activity is regulated by autoinhibition, positive feedback, and interaction with other GTPases. Although these mechanisms have been characterized biochemically, we lack a clear picture of how GEF localization and activity is modulated by these signals. Here, we report the cryogenic electron microscopy structure of full-length Sec7 in its autoinhibited form, revealing the architecture of its multiple regulatory domains. We use functional experiments to determine the basis for autoinhibition and use structural predictions to produce a model for an active conformation of the GEF that is supported empirically. This study therefore elucidates the conformational transition that Sec7 undergoes to become active on the organelle membrane surface. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42183.map.gz emd_42183.map.gz | 1.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42183-v30.xml emd-42183-v30.xml emd-42183.xml emd-42183.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42183.png emd_42183.png | 24.5 KB | ||
| Filedesc metadata |  emd-42183.cif.gz emd-42183.cif.gz | 6.5 KB | ||
| Others |  emd_42183_half_map_1.map.gz emd_42183_half_map_1.map.gz emd_42183_half_map_2.map.gz emd_42183_half_map_2.map.gz | 337.4 MB 337.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42183 http://ftp.pdbj.org/pub/emdb/structures/EMD-42183 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42183 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42183 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_42183.map.gz / Format: CCP4 / Size: 440.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42183.map.gz / Format: CCP4 / Size: 440.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
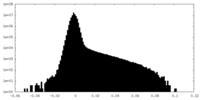
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.7415 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_42183_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
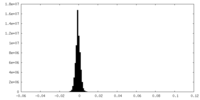
| Density Histograms |
-Half map: #2
| File | emd_42183_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
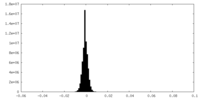
| Density Histograms |
- Sample components
Sample components
-Entire : Sec7 homodimer
| Entire | Name: Sec7 homodimer |
|---|---|
| Components |
|
-Supramolecule #1: Sec7 homodimer
| Supramolecule | Name: Sec7 homodimer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Thermothielavioides terrestris (fungus) Thermothielavioides terrestris (fungus) |
| Molecular weight | Theoretical: 392.14 KDa |
-Macromolecule #1: SEC7 domain-containing protein
| Macromolecule | Name: SEC7 domain-containing protein / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Thermothielavioides terrestris (fungus) Thermothielavioides terrestris (fungus) |
| Recombinant expression | Organism:  Komagataella pastoris (fungus) Komagataella pastoris (fungus) |
| Sequence | String: MEQKLISEED LNSAVDHHHH HHRIPGLINS SLKFVVSSLD IIAAQAGRNK QLAELAEKAL AAIKENDQQL PDPEVVFAPL QLATKSGTIP LTTTALDCIG KLISYSYFSA PSSSATQDGT EQTPLIERAI DTICDCFQGE TTLVEIQLQI VKSLLAAVLN DKIIVHGAGL ...String: MEQKLISEED LNSAVDHHHH HHRIPGLINS SLKFVVSSLD IIAAQAGRNK QLAELAEKAL AAIKENDQQL PDPEVVFAPL QLATKSGTIP LTTTALDCIG KLISYSYFSA PSSSATQDGT EQTPLIERAI DTICDCFQGE TTLVEIQLQI VKSLLAAVLN DKIIVHGAGL LKAVRQVYNI FLLSRSTANQ QVAQGTLTQM VGTVFERVKT RLHMKEARAN LGRLKASRSS LAVDRSDDQD SQAGKVDGED ATVETVSDAT PSESVDKAGG GKLTLKDLEH RKSFDDSHMG DGPTMVSQVK PMKKASRSVS EQSLQESPQD ETPESLDAED EAYIRDAYLV FRSFCNLSTK ILPPDQLYDL RGQPMRSKLI SLHLIHTLLN NHITVFTSPL CTIRNTKNNE PTNFLQAIKY YLCLSITRNG ASSVDRVFDI CCEIFWLMLK YMRSSFKNEI EVFLNEIYLA LLARRNAPLS QKLTFVGILK RLCEDPRALV ELYLNYDCNQ NVDNIFQTIV EDLSRFATAS VPITPTQEQQ YEESRSKSAT AGEWQIKSVL PPPLSVALIA TNHEADTELP KEYVMKRTAL DSLVETLRSL VHWSQPGRPE LNGASGDVQR RTSSDDLGDS IDPSMSETAS RMEVPIAPAT PVIDDDPDQL EKEKARKTAM TNAIKVFNFK PKHGIKLLIK EGFIPSDKPE DIARFLLREE RLDKAQIGEY LGEGDQKNVD IMHAFVDMMD FSKKRFVDAL REFLQAFRLP GEAQKIDRFM LKFAHRYVTG NPNAFANADT PYVLAYSVIM LNTDLHSSKV VKRMSKAEFI KNNRGINDNA DLPDEYLIGI YDDIASNEIV LKSEREAAAA AGTLPAQSTG LAGLGQAFSN VGRDLQREAY VQQSEEISLR SEQLFRDLYR SQRKSATKGG VKFISATSFK HVGPMFDATW MSFFSTLSSL VQKTHNLDVN KLCLEGMKLA TKIACLFDLS TPREAFISML KNTANLNNPR EMQAKNVEAL KVLLDLAQTE GNYLKESWKD VLLCISQLDR LQLISGGVDE SAVPDVSRAR FVPPPRTETG ESRKSTSSAR RTRPRAHTGP QGVSLEIALE SRSDEVIKSV DRIFTNTANL SRDAIIHFAR ALTEVSWDEI KVSGSNDSPR TYSLQKIVEI SYYNMTRVRF EWSHIWDVLG EHFNRVGCHA NTAIVFFALD SLRQLSMRFM EIEELAGFKF QKDFLKPFEH VMSNSSNVTV KDMVLRCLIQ MIQARGENIR SGWRTMFGVF TVAAREPYES IVNLAYENVT QVYKTRFGVV ISQGAFTDLI VCLTEFSKNM RFQKKSLQAM ETLKSVIPTM LKTPECPLSQ HKPTATTASG SESHSKKAAV QQTRTSVEEG FWFPVLFAFH DVLMTGEDLE VRSNALNYFF ETLLRYGGDF PPEFWDILWR QQLYPIFMVL RSRPEMTNAL NHEELSVWLS TTMIQALRNM ITLFTHYFDA LEYMLDRFLE LLALCICQEN DTIARIGSNC LQQLILQNVT KFTAEHWAKI VGAFCELFER TTAYQLFSAT TINSTASLSP PPSGLELGGP LSPTSATAPV DGKSLKINGV ETNGQTPGAE PANGDADGNG TAAAAADASA PAATPQPQQG PAQQLEEFKP NNPLQQQPVV VTAARRRFFN RIISRCVLQL LMIETVNELF SNDAVYAQIP SAELLRLMAL LKKSFLFAKR FNADKDLRMR LWREGFMKQP PNLLKQESGS AATYVAILFR MFGDTAPDRR GSRADVEAAL VPLCRDIIRG YTALDDESQH RNIVAWRPVV VDVLEGYAAF PRDAFAAHIR SFYPLVVELL GKDLGQDLRA ALLLVLRRVG EVGLGIEGMG SGGAAAAAAA GAAAASSGQG NGNGAAAAAA DSERRSSVLS VPSGPRHTPS MDSLNDDPSR QVMGKAEQKL ISEEDLNSAV DHHHHHH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8.0 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 4474 / Average exposure time: 3.092 sec. / Average electron dose: 55.6 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 63000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Details | Phenix Real Space Refinement |
| Refinement | Space: REAL / Protocol: OTHER |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)