[English] 日本語
 Yorodumi
Yorodumi- EMDB-41922: Cryo-EM structure of human DNMT3A UDR bound to H2AK119ub1-modifie... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human DNMT3A UDR bound to H2AK119ub1-modified nucleosome | |||||||||
 Map data Map data | main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA cytosine methyltransferase / STRUCTURAL PROTEIN-TRANSFERASE-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of cellular response to hypoxia / transposable element silencing by piRNA-mediated DNA methylation / protein-cysteine methyltransferase activity / regulatory ncRNA-mediated heterochromatin formation / unmethylated CpG binding / cellular response to bisphenol A / DNA (cytosine-5-)-methyltransferase / DNA (cytosine-5-)-methyltransferase activity / autosome genomic imprinting / SUMOylation of DNA methylation proteins ...positive regulation of cellular response to hypoxia / transposable element silencing by piRNA-mediated DNA methylation / protein-cysteine methyltransferase activity / regulatory ncRNA-mediated heterochromatin formation / unmethylated CpG binding / cellular response to bisphenol A / DNA (cytosine-5-)-methyltransferase / DNA (cytosine-5-)-methyltransferase activity / autosome genomic imprinting / SUMOylation of DNA methylation proteins / XY body / response to vitamin A / DNA methylation-dependent constitutive heterochromatin formation / negative regulation of gene expression via chromosomal CpG island methylation / hepatocyte apoptotic process / response to ionizing radiation / lncRNA binding / cellular response to ethanol / chromosome, centromeric region / catalytic complex / heterochromatin / Maturation of protein E / Maturation of protein E / ER Quality Control Compartment (ERQC) / Myoclonic epilepsy of Lafora / FLT3 signaling by CBL mutants / Constitutive Signaling by NOTCH1 HD Domain Mutants / IRAK2 mediated activation of TAK1 complex / Prevention of phagosomal-lysosomal fusion / Alpha-protein kinase 1 signaling pathway / Glycogen synthesis / IRAK1 recruits IKK complex / IRAK1 recruits IKK complex upon TLR7/8 or 9 stimulation / Endosomal Sorting Complex Required For Transport (ESCRT) / Membrane binding and targetting of GAG proteins / Negative regulation of FLT3 / Regulation of TBK1, IKKε (IKBKE)-mediated activation of IRF3, IRF7 / PTK6 Regulates RTKs and Their Effectors AKT1 and DOK1 / Regulation of TBK1, IKKε-mediated activation of IRF3, IRF7 upon TLR3 ligation / IRAK2 mediated activation of TAK1 complex upon TLR7/8 or 9 stimulation / NOTCH2 Activation and Transmission of Signal to the Nucleus / TICAM1,TRAF6-dependent induction of TAK1 complex / TICAM1-dependent activation of IRF3/IRF7 / APC/C:Cdc20 mediated degradation of Cyclin B / Regulation of FZD by ubiquitination / Downregulation of ERBB4 signaling / APC-Cdc20 mediated degradation of Nek2A / p75NTR recruits signalling complexes / InlA-mediated entry of Listeria monocytogenes into host cells / TRAF6 mediated IRF7 activation in TLR7/8 or 9 signaling / Regulation of pyruvate metabolism / TRAF6-mediated induction of TAK1 complex within TLR4 complex / NF-kB is activated and signals survival / Regulation of innate immune responses to cytosolic DNA / Pexophagy / Downregulation of ERBB2:ERBB3 signaling / NRIF signals cell death from the nucleus / Transferases; Transferring one-carbon groups; Methyltransferases / Activated NOTCH1 Transmits Signal to the Nucleus / Regulation of PTEN localization / VLDLR internalisation and degradation / Synthesis of active ubiquitin: roles of E1 and E2 enzymes / Regulation of BACH1 activity / DNA methylation / Translesion synthesis by REV1 / TICAM1, RIP1-mediated IKK complex recruitment / MAP3K8 (TPL2)-dependent MAPK1/3 activation / Translesion synthesis by POLK / InlB-mediated entry of Listeria monocytogenes into host cell / JNK (c-Jun kinases) phosphorylation and activation mediated by activated human TAK1 / Activation of IRF3, IRF7 mediated by TBK1, IKKε (IKBKE) / Josephin domain DUBs / Downregulation of TGF-beta receptor signaling / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / IKK complex recruitment mediated by RIP1 / Regulation of activated PAK-2p34 by proteasome mediated degradation / post-embryonic development / PINK1-PRKN Mediated Mitophagy / PRC2 methylates histones and DNA / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / TNFR1-induced NF-kappa-B signaling pathway / response to cocaine / Defective pyroptosis / Autodegradation of Cdh1 by Cdh1:APC/C / TCF dependent signaling in response to WNT / APC/C:Cdc20 mediated degradation of Securin / Regulation of NF-kappa B signaling / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / activated TAK1 mediates p38 MAPK activation / Asymmetric localization of PCP proteins / Ubiquitin-dependent degradation of Cyclin D / SCF-beta-TrCP mediated degradation of Emi1 / NIK-->noncanonical NF-kB signaling / cellular response to amino acid stimulus / TNFR2 non-canonical NF-kB pathway / Regulation of signaling by CBL / AUF1 (hnRNP D0) binds and destabilizes mRNA / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / NOTCH3 Activation and Transmission of Signal to the Nucleus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) / | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.23 Å | |||||||||
 Authors Authors | Chen X / Song J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis for the H2AK119ub1-specific DNMT3A-nucleosome interaction. Authors: Xinyi Chen / Yiran Guo / Ting Zhao / Jiuwei Lu / Jian Fang / Yinsheng Wang / Gang Greg Wang / Jikui Song /  Abstract: Isoform 1 of DNA methyltransferase DNMT3A (DNMT3A1) specifically recognizes nucleosome monoubiquitylated at histone H2A lysine-119 (H2AK119ub1) for establishment of DNA methylation. Mis-regulation of ...Isoform 1 of DNA methyltransferase DNMT3A (DNMT3A1) specifically recognizes nucleosome monoubiquitylated at histone H2A lysine-119 (H2AK119ub1) for establishment of DNA methylation. Mis-regulation of this process may cause aberrant DNA methylation and pathogenesis. However, the molecular basis underlying DNMT3A1-nucleosome interaction remains elusive. Here we report the cryo-EM structure of DNMT3A1's ubiquitin-dependent recruitment (UDR) fragment complexed with H2AK119ub1-modified nucleosome. DNMT3A1 UDR occupies an extensive nucleosome surface, involving the H2A-H2B acidic patch, a surface groove formed by H2A and H3, nucleosomal DNA, and H2AK119ub1. The DNMT3A1 UDR's interaction with H2AK119ub1 affects the functionality of DNMT3A1 in cells in a context-dependent manner. Our structural and biochemical analysis also reveals competition between DNMT3A1 and JARID2, a cofactor of polycomb repression complex 2 (PRC2), for nucleosome binding, suggesting the interplay between different epigenetic pathways. Together, this study reports a molecular basis for H2AK119ub1-dependent DNMT3A1-nucleosome association, with important implications in DNMT3A1-mediated DNA methylation in development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41922.map.gz emd_41922.map.gz | 25.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41922-v30.xml emd-41922-v30.xml emd-41922.xml emd-41922.xml | 22.5 KB 22.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41922.png emd_41922.png | 102.3 KB | ||
| Filedesc metadata |  emd-41922.cif.gz emd-41922.cif.gz | 6.9 KB | ||
| Others |  emd_41922_additional_1.map.gz emd_41922_additional_1.map.gz | 12.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41922 http://ftp.pdbj.org/pub/emdb/structures/EMD-41922 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41922 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41922 | HTTPS FTP |
-Related structure data
| Related structure data |  8u5hMC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41922.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41922.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.2347 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: additional map
| File | emd_41922_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | additional map | ||||||||||||
| Projections & Slices |
| ||||||||||||
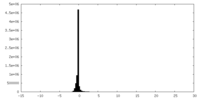
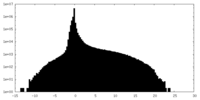
| Density Histograms |
- Sample components
Sample components
-Entire : DNMT3A N-terminal domain and H2AK119Ub nucleosome complex
| Entire | Name: DNMT3A N-terminal domain and H2AK119Ub nucleosome complex |
|---|---|
| Components |
|
-Supramolecule #1: DNMT3A N-terminal domain and H2AK119Ub nucleosome complex
| Supramolecule | Name: DNMT3A N-terminal domain and H2AK119Ub nucleosome complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#8 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: DNA (cytosine-5)-methyltransferase 3A
| Macromolecule | Name: DNA (cytosine-5)-methyltransferase 3A / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.070631 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PEASRAVENG CCTPKEGRGA PAEAGKEQKE TNIESMKMEG SRGRLRGGLG WESSLRQRPM PRLTFQAGDP YYISKRKRDE WLARWKREA EKKAKVIAG UniProtKB: DNA (cytosine-5)-methyltransferase 3A |
-Macromolecule #3: Ubiquitin
| Macromolecule | Name: Ubiquitin / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 8.576831 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQIFVKTLTG KTITLEVEPS DTIENVKAKI QDKEGIPPDQ QRLIFAGKQL EDGRTLSDYN IQKESTLHLV LRLRGG UniProtKB: Polyubiquitin-C |
-Macromolecule #5: Histone H3.3C
| Macromolecule | Name: Histone H3.3C / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 15.521349 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LVTKAAKKCA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQRSA VMALQEASEA YLVALFEDTN LCAIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3.3C |
-Macromolecule #6: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 6 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #7: Histone H2A
| Macromolecule | Name: Histone H2A / type: protein_or_peptide / ID: 7 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 14.083398 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKQGGK TRAKAKTRSS RAGLQFPVGR VHRLLRKGNY AERVGAGAPV YLAAVLEYLT AEILELAGNA ARDNKKTRII PRHLQLAVR NDEELNKLLG RVTIAQGGVL PNIQSVLLPK CTESSKSAKS K UniProtKB: Histone H2A |
-Macromolecule #8: Histone H2B 1.1
| Macromolecule | Name: Histone H2B 1.1 / type: protein_or_peptide / ID: 8 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 13.655948 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAKSAPAPKK GSKKAVTKTQ KKDGKKRRKT RKESYAIYVY KVLKQVHPDT GISSKAMSIM NSFVNDVFER IAGEASRLAH YNKRSTITS REIQTAVRLL LPGELAKHAV SEGTKAVTKY TSAK UniProtKB: Histone H2B 1.1 |
-Macromolecule #2: nucleosome DNA
| Macromolecule | Name: nucleosome DNA / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 55.176137 KDa |
| Sequence | String: (DG)(DC)(DT)(DC)(DT)(DC)(DT)(DA)(DC)(DG) (DT)(DA)(DA)(DA)(DC)(DA)(DT)(DC)(DC)(DT) (DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC)(DC) (DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA)(DG) (DG) (DC)(DC)(DG)(DC)(DT)(DC) ...String: (DG)(DC)(DT)(DC)(DT)(DC)(DT)(DA)(DC)(DG) (DT)(DA)(DA)(DA)(DC)(DA)(DT)(DC)(DC)(DT) (DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC)(DC) (DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA)(DG) (DG) (DC)(DC)(DG)(DC)(DT)(DC)(DA)(DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA)(DG) (DA)(DC) (DA)(DG)(DC)(DT)(DC)(DT)(DA) (DG)(DC)(DA)(DC)(DC)(DG)(DC)(DT)(DT)(DA) (DA)(DA)(DC) (DG)(DC)(DA)(DC)(DG)(DT) (DA)(DC)(DG)(DC)(DG)(DC)(DT)(DG)(DT)(DC) (DC)(DC)(DC)(DC) (DG)(DC)(DG)(DT)(DT) (DT)(DT)(DA)(DA)(DC)(DC)(DG)(DC)(DC)(DA) (DA)(DG)(DG)(DG)(DG) (DA)(DT)(DT)(DA) (DC)(DT)(DC)(DC)(DC)(DT)(DA)(DG)(DT)(DC) (DT)(DC)(DC)(DA)(DG)(DG) (DC)(DA)(DC) (DG)(DT)(DG)(DT)(DC)(DA)(DG)(DA)(DT)(DA) (DT)(DA)(DT)(DA)(DC)(DA)(DT) (DC)(DC) (DT)(DG)(DT)(DG)(DA)(DC)(DT)(DT)(DA)(DC) (DC)(DC)(DA)(DC)(DT)(DT)(DC)(DG) |
-Macromolecule #4: nucleosome DNA
| Macromolecule | Name: nucleosome DNA / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 55.963656 KDa |
| Sequence | String: (DC)(DG)(DA)(DA)(DG)(DT)(DG)(DG)(DG)(DT) (DA)(DA)(DG)(DT)(DC)(DA)(DC)(DA)(DG)(DG) (DA)(DT)(DG)(DT)(DA)(DT)(DA)(DT)(DA) (DT)(DC)(DT)(DG)(DA)(DC)(DA)(DC)(DG)(DT) (DG) (DC)(DC)(DT)(DG)(DG)(DA) ...String: (DC)(DG)(DA)(DA)(DG)(DT)(DG)(DG)(DG)(DT) (DA)(DA)(DG)(DT)(DC)(DA)(DC)(DA)(DG)(DG) (DA)(DT)(DG)(DT)(DA)(DT)(DA)(DT)(DA) (DT)(DC)(DT)(DG)(DA)(DC)(DA)(DC)(DG)(DT) (DG) (DC)(DC)(DT)(DG)(DG)(DA)(DG)(DA) (DC)(DT)(DA)(DG)(DG)(DG)(DA)(DG)(DT)(DA) (DA)(DT) (DC)(DC)(DC)(DC)(DT)(DT)(DG) (DG)(DC)(DG)(DG)(DT)(DT)(DA)(DA)(DA)(DA) (DC)(DG)(DC) (DG)(DG)(DG)(DG)(DG)(DA) (DC)(DA)(DG)(DC)(DG)(DC)(DG)(DT)(DA)(DC) (DG)(DT)(DG)(DC) (DG)(DT)(DT)(DT)(DA) (DA)(DG)(DC)(DG)(DG)(DT)(DG)(DC)(DT)(DA) (DG)(DA)(DG)(DC)(DT) (DG)(DT)(DC)(DT) (DA)(DC)(DG)(DA)(DC)(DC)(DA)(DA)(DT)(DT) (DG)(DA)(DG)(DC)(DG)(DG) (DC)(DC)(DT) (DC)(DG)(DG)(DC)(DA)(DC)(DC)(DG)(DG)(DG) (DA)(DT)(DT)(DC)(DT)(DC)(DC) (DA)(DG) (DG)(DA)(DT)(DG)(DT)(DT)(DT)(DA)(DC)(DG) (DT)(DA)(DG)(DA)(DG)(DA)(DG)(DC) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

























 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)