+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-4146 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
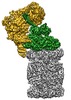
| タイトル | Human 26S proteasome in complex with Oprozomib | ||||||||||||
 マップデータ マップデータ | |||||||||||||
 試料 試料 |
| ||||||||||||
 キーワード キーワード | proteasome / oprozomib / ups / drug-binding / Hydrolase | ||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Impaired BRCA2 translocation to the nucleus / Impaired BRCA2 binding to SEM1 (DSS1) / thyrotropin-releasing hormone receptor binding / nuclear proteasome complex / host-mediated perturbation of viral transcription / positive regulation of inclusion body assembly / 加水分解酵素; プロテアーゼ; ペプチド結合加水分解酵素; オメガペプチターゼ / integrator complex / proteasome accessory complex / meiosis I ...Impaired BRCA2 translocation to the nucleus / Impaired BRCA2 binding to SEM1 (DSS1) / thyrotropin-releasing hormone receptor binding / nuclear proteasome complex / host-mediated perturbation of viral transcription / positive regulation of inclusion body assembly / 加水分解酵素; プロテアーゼ; ペプチド結合加水分解酵素; オメガペプチターゼ / integrator complex / proteasome accessory complex / meiosis I / purine ribonucleoside triphosphate binding / proteasome regulatory particle / cytosolic proteasome complex / positive regulation of proteasomal protein catabolic process / proteasome-activating activity / proteasome regulatory particle, lid subcomplex / proteasome regulatory particle, base subcomplex / protein K63-linked deubiquitination / negative regulation of programmed cell death / metal-dependent deubiquitinase activity / Regulation of ornithine decarboxylase (ODC) / Proteasome assembly / Cross-presentation of soluble exogenous antigens (endosomes) / proteasome core complex / Homologous DNA Pairing and Strand Exchange / Defective homologous recombination repair (HRR) due to BRCA1 loss of function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA1 binding function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA2/RAD51/RAD51C binding function / Resolution of D-loop Structures through Synthesis-Dependent Strand Annealing (SDSA) / Somitogenesis / Resolution of D-loop Structures through Holliday Junction Intermediates / K63-linked deubiquitinase activity / Impaired BRCA2 binding to RAD51 / proteasome binding / transcription factor binding / regulation of protein catabolic process / myofibril / proteasome storage granule / Presynaptic phase of homologous DNA pairing and strand exchange / general transcription initiation factor binding / blastocyst development / polyubiquitin modification-dependent protein binding / positive regulation of RNA polymerase II transcription preinitiation complex assembly / protein deubiquitination / immune system process / NF-kappaB binding / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / endopeptidase activator activity / proteasome assembly / threonine-type endopeptidase activity / mRNA export from nucleus / proteasome core complex, alpha-subunit complex / SARS-CoV-1 targets host intracellular signalling and regulatory pathways / enzyme regulator activity / regulation of proteasomal protein catabolic process / ERAD pathway / inclusion body / proteasome complex / TBP-class protein binding / proteolysis involved in protein catabolic process / sarcomere / Regulation of activated PAK-2p34 by proteasome mediated degradation / Autodegradation of Cdh1 by Cdh1:APC/C / APC/C:Cdc20 mediated degradation of Securin / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / Asymmetric localization of PCP proteins / Ubiquitin-dependent degradation of Cyclin D / SCF-beta-TrCP mediated degradation of Emi1 / NIK-->noncanonical NF-kB signaling / stem cell differentiation / TNFR2 non-canonical NF-kB pathway / AUF1 (hnRNP D0) binds and destabilizes mRNA / Vpu mediated degradation of CD4 / Assembly of the pre-replicative complex / Ubiquitin-Mediated Degradation of Phosphorylated Cdc25A / Degradation of DVL / Dectin-1 mediated noncanonical NF-kB signaling / negative regulation of inflammatory response to antigenic stimulus / Cdc20:Phospho-APC/C mediated degradation of Cyclin A / lipopolysaccharide binding / P-body / Degradation of AXIN / Hh mutants are degraded by ERAD / Activation of NF-kappaB in B cells / Degradation of GLI1 by the proteasome / G2/M Checkpoints / Hedgehog ligand biogenesis / Defective CFTR causes cystic fibrosis / Autodegradation of the E3 ubiquitin ligase COP1 / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Regulation of RUNX3 expression and activity / Negative regulation of NOTCH4 signaling / Hedgehog 'on' state / Vif-mediated degradation of APOBEC3G / APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1 / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / double-strand break repair via homologous recombination 類似検索 - 分子機能 | ||||||||||||
| 生物種 |  Homo sapiens (ヒト) / Synthetic construct (人工物) Homo sapiens (ヒト) / Synthetic construct (人工物) | ||||||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.8 Å | ||||||||||||
 データ登録者 データ登録者 | Haselbach D / Schrader J | ||||||||||||
| 資金援助 |  ドイツ, 3件 ドイツ, 3件
| ||||||||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2017 ジャーナル: Nat Commun / 年: 2017タイトル: Long-range allosteric regulation of the human 26S proteasome by 20S proteasome-targeting cancer drugs. 著者: David Haselbach / Jil Schrader / Felix Lambrecht / Fabian Henneberg / Ashwin Chari / Holger Stark /  要旨: The proteasome holoenzyme is the major non-lysosomal protease; its proteolytic activity is essential for cellular homeostasis. Thus, it is an attractive target for the development of ...The proteasome holoenzyme is the major non-lysosomal protease; its proteolytic activity is essential for cellular homeostasis. Thus, it is an attractive target for the development of chemotherapeutics. While the structural basis of core particle (CP) inhibitors is largely understood, their structural impact on the proteasome holoenzyme remains entirely elusive. Here, we determined the structure of the 26S proteasome with and without the inhibitor Oprozomib. Drug binding modifies the energy landscape of conformational motion in the proteasome regulatory particle (RP). Structurally, the energy barrier created by Oprozomib triggers a long-range allosteric regulation, resulting in the stabilization of a non-productive state. Thereby, the chemical drug-binding signal is converted, propagated and amplified into structural changes over a distance of more than 150 Å from the proteolytic site to the ubiquitin receptor Rpn10. The direct visualization of changes in conformational dynamics upon drug binding allows new ways to screen and develop future allosteric proteasome inhibitors. | ||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_4146.map.gz emd_4146.map.gz | 13.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-4146-v30.xml emd-4146-v30.xml emd-4146.xml emd-4146.xml | 64.1 KB 64.1 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_4146.png emd_4146.png | 150.4 KB | ||
| Filedesc metadata |  emd-4146.cif.gz emd-4146.cif.gz | 14.6 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4146 http://ftp.pdbj.org/pub/emdb/structures/EMD-4146 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4146 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4146 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_4146_validation.pdf.gz emd_4146_validation.pdf.gz | 411 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_4146_full_validation.pdf.gz emd_4146_full_validation.pdf.gz | 410.5 KB | 表示 | |
| XML形式データ |  emd_4146_validation.xml.gz emd_4146_validation.xml.gz | 6.8 KB | 表示 | |
| CIF形式データ |  emd_4146_validation.cif.gz emd_4146_validation.cif.gz | 7.8 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4146 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4146 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4146 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4146 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  5m32MC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | |
| 電子顕微鏡画像生データ |  EMPIAR-10166 (タイトル: human 26S proteasome bound to the chemotherapeutic Oprozomib EMPIAR-10166 (タイトル: human 26S proteasome bound to the chemotherapeutic OprozomibData size: 100.5 Data #1: Particle stack of aligned, summed and dose weighted particle images from the human 26S proteasome bound to oprozomib. [picked particles - single frame - processed]) |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_4146.map.gz / 形式: CCP4 / 大きさ: 144.7 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_4146.map.gz / 形式: CCP4 / 大きさ: 144.7 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.27 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
+全体 : human 26S proteasome in complex with oprozomib
+超分子 #1: human 26S proteasome in complex with oprozomib
+超分子 #2: human 26S proteasome
+超分子 #3: bound oprozomib
+分子 #1: Proteasome subunit alpha type-2
+分子 #2: Proteasome subunit alpha type-4
+分子 #3: Proteasome subunit alpha type-7
+分子 #4: Proteasome subunit alpha type-5
+分子 #5: Proteasome subunit alpha type-1
+分子 #6: Proteasome subunit alpha type-3
+分子 #7: Proteasome subunit alpha type-6
+分子 #8: Proteasome subunit beta type-7
+分子 #9: Proteasome subunit beta type-3
+分子 #10: Proteasome subunit beta type-2
+分子 #11: Proteasome subunit beta type-5
+分子 #12: Proteasome subunit beta type-1
+分子 #13: Proteasome subunit beta type-4
+分子 #14: Proteasome subunit beta type-6
+分子 #15: Proteasome subunit alpha type-7
+分子 #16: Proteasome subunit alpha type-1
+分子 #17: 26S protease regulatory subunit 7
+分子 #18: 26S protease regulatory subunit 4
+分子 #19: 26S protease regulatory subunit 6B
+分子 #20: 26S protease regulatory subunit 10B
+分子 #21: 26S protease regulatory subunit 6A
+分子 #22: 26S protease regulatory subunit 8
+分子 #23: 26S proteasome non-ATPase regulatory subunit 1
+分子 #24: 26S proteasome non-ATPase regulatory subunit 3
+分子 #25: 26S proteasome non-ATPase regulatory subunit 12
+分子 #26: 26S proteasome non-ATPase regulatory subunit 11
+分子 #27: 26S proteasome non-ATPase regulatory subunit 6
+分子 #28: 26S proteasome non-ATPase regulatory subunit 7
+分子 #29: 26S proteasome non-ATPase regulatory subunit 13
+分子 #30: 26S proteasome non-ATPase regulatory subunit 4
+分子 #31: 26S proteasome non-ATPase regulatory subunit 14
+分子 #32: 26S proteasome non-ATPase regulatory subunit 8
+分子 #33: 26S proteasome complex subunit SEM1
+分子 #34: bound Oprozomib
+分子 #35: ADENOSINE-5'-DIPHOSPHATE
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 6.5 構成要素:
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| グリッド | モデル: Quantifoil / 材質: COPPER / メッシュ: 200 / 支持フィルム - #0 - Film type ID: 1 / 支持フィルム - #0 - 材質: CARBON / 支持フィルム - #0 - トポロジー: HOLEY / 支持フィルム - #1 - Film type ID: 2 / 支持フィルム - #1 - 材質: CARBON / 支持フィルム - #1 - トポロジー: CONTINUOUS | ||||||||||||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 72 % / チャンバー内温度: 4 K / 装置: LEICA EM GP / 詳細: blot with sensor. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 特殊光学系 | 球面収差補正装置: Microscope is equipped with Cs corrector |
| 撮影 | フィルム・検出器のモデル: FEI FALCON II (4k x 4k) 検出モード: OTHER / デジタル化 - サイズ - 横: 4096 pixel / デジタル化 - サイズ - 縦: 4096 pixel / 撮影したグリッド数: 1 / 平均露光時間: 1.0 sec. / 平均電子線量: 2.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 70.0 µm / 倍率(補正後): 110236 / 照射モード: SPOT SCAN / 撮影モード: BRIGHT FIELD / Cs: 0.0001 mm / 最大 デフォーカス(公称値): 8.4 µm / 最小 デフォーカス(公称値): 0.3 µm / 倍率(公称値): 59000 |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















