[English] 日本語
 Yorodumi
Yorodumi- EMDB-41436: Central rod disk in D3 symmetry of high-resolution phycobilisome ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Central rod disk in D3 symmetry of high-resolution phycobilisome quenched by OCP (local refinement) | |||||||||||||||
 Map data Map data | sharpened map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Complex / light harvesting / pigment / PHOTOSYNTHESIS | |||||||||||||||
| Biological species |   | |||||||||||||||
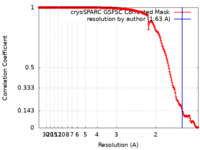
| Method | single particle reconstruction / cryo EM / Resolution: 1.63 Å | |||||||||||||||
 Authors Authors | Sauer PV / Sutter M / Cupellini L / Kirst H / Kerfeld CA | |||||||||||||||
| Funding support | European Union,  United States, United States,  Czech Republic, 4 items Czech Republic, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2024 Journal: Sci Adv / Year: 2024Title: Structural and quantum chemical basis for OCP-mediated quenching of phycobilisomes. Authors: Paul V Sauer / Lorenzo Cupellini / Markus Sutter / Mattia Bondanza / María Agustina Domínguez Martin / Henning Kirst / David Bína / Adrian Fujiet Koh / Abhay Kotecha / Basil J Greber / ...Authors: Paul V Sauer / Lorenzo Cupellini / Markus Sutter / Mattia Bondanza / María Agustina Domínguez Martin / Henning Kirst / David Bína / Adrian Fujiet Koh / Abhay Kotecha / Basil J Greber / Eva Nogales / Tomáš Polívka / Benedetta Mennucci / Cheryl A Kerfeld /      Abstract: Cyanobacteria use large antenna complexes called phycobilisomes (PBSs) for light harvesting. However, intense light triggers non-photochemical quenching, where the orange carotenoid protein (OCP) ...Cyanobacteria use large antenna complexes called phycobilisomes (PBSs) for light harvesting. However, intense light triggers non-photochemical quenching, where the orange carotenoid protein (OCP) binds to PBS, dissipating excess energy as heat. The mechanism of efficiently transferring energy from phycocyanobilins in PBS to canthaxanthin in OCP remains insufficiently understood. Using cryo-electron microscopy, we unveiled the OCP-PBS complex structure at 1.6- to 2.1-angstrom resolution, showcasing its inherent flexibility. Using multiscale quantum chemistry, we disclosed the quenching mechanism. Identifying key protein residues, we clarified how canthaxanthin's transition dipole moment in its lowest-energy dark state becomes large enough for efficient energy transfer from phycocyanobilins. Our energy transfer model offers a detailed understanding of the atomic determinants of light harvesting regulation and antenna architecture in cyanobacteria. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41436.map.gz emd_41436.map.gz | 483.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41436-v30.xml emd-41436-v30.xml emd-41436.xml emd-41436.xml | 18.4 KB 18.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41436_fsc.xml emd_41436_fsc.xml | 16.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_41436.png emd_41436.png | 84.7 KB | ||
| Masks |  emd_41436_msk_1.map emd_41436_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-41436.cif.gz emd-41436.cif.gz | 5.1 KB | ||
| Others |  emd_41436_additional_1.map.gz emd_41436_additional_1.map.gz emd_41436_half_map_1.map.gz emd_41436_half_map_1.map.gz emd_41436_half_map_2.map.gz emd_41436_half_map_2.map.gz | 258.3 MB 475.4 MB 475.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41436 http://ftp.pdbj.org/pub/emdb/structures/EMD-41436 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41436 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41436 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41436.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41436.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.727 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_41436_msk_1.map emd_41436_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: refined map
| File | emd_41436_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | refined map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_41436_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_41436_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of phycobilisome from Synechocystis PCC 6803 bound to OCP
| Entire | Name: Complex of phycobilisome from Synechocystis PCC 6803 bound to OCP |
|---|---|
| Components |
|
-Supramolecule #1: Complex of phycobilisome from Synechocystis PCC 6803 bound to OCP
| Supramolecule | Name: Complex of phycobilisome from Synechocystis PCC 6803 bound to OCP type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: C-phycocyanin alpha subunit
| Macromolecule | Name: C-phycocyanin alpha subunit / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MKTPLTEAVS TADSQGRFLS STELQIAFGR LRQANAGLQA AKALTDNAQS LVNGAAQAVY NKFPYTTQTQ GNNFAADQRG KDKCARDIGY YLRIVTYCLV AGGTGPLDEY LIAGIDEINR TFDLSPSWYV EALKYIKANH GLSGDARDEA NSYLDYAINA LS |
-Macromolecule #2: C-phycocyanin beta subunit
| Macromolecule | Name: C-phycocyanin beta subunit / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MFDVFTRVVS QADARGEYLS GSQLDALSAT VAEGNKRIDS VNRITGNASA IVSNAARALF AEQPQLIQPG GNAYTSRRMA ACLRDMEIIL RYVTYATFTG DASVLEDRCL NGLRETYVAL GVPGASVAAG VQKMKEAALD IVNDPNGITR GDCSAIVAEI AGYFDRAAAA VA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.8 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE-PROPANE / Instrument: FEI VITROBOT MARK IV / Details: Manual blotting. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.4 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)