[English] 日本語
 Yorodumi
Yorodumi- EMDB-41428: Substrate Binding Plasticity Revealed by Cryo-EM Structures of SLC26A2 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Substrate Binding Plasticity Revealed by Cryo-EM Structures of SLC26A2 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | SLC26A2 / sulfate transporter / MEMBRANE PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationDefective SLC26A2 causes chondrodysplasias / Transport and metabolism of PAPS / Inorganic anion exchange by SLC26 transporters / sulfate transmembrane transporter activity / oxalate transmembrane transporter activity / chondrocyte proliferation / sulfate transmembrane transport / secondary active sulfate transmembrane transporter activity / solute:inorganic anion antiporter activity / bicarbonate transmembrane transporter activity ...Defective SLC26A2 causes chondrodysplasias / Transport and metabolism of PAPS / Inorganic anion exchange by SLC26 transporters / sulfate transmembrane transporter activity / oxalate transmembrane transporter activity / chondrocyte proliferation / sulfate transmembrane transport / secondary active sulfate transmembrane transporter activity / solute:inorganic anion antiporter activity / bicarbonate transmembrane transporter activity / chloride transmembrane transporter activity / microvillus membrane / chondrocyte differentiation / chloride transmembrane transport / apical plasma membrane / extracellular exosome / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.03 Å | ||||||||||||
 Authors Authors | Hu W / Song A | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Substrate binding plasticity revealed by Cryo-EM structures of SLC26A2. Authors: Wenxin Hu / Alex Song / Hongjin Zheng Abstract: SLC26A2 is a vital solute carrier responsible for transporting essential nutritional ions, including sulfate, within the human body. Pathogenic mutations within SLC26A2 give rise to a spectrum of ...SLC26A2 is a vital solute carrier responsible for transporting essential nutritional ions, including sulfate, within the human body. Pathogenic mutations within SLC26A2 give rise to a spectrum of human diseases, ranging from lethal to mild symptoms. The molecular details regarding the versatile substrate-transporter interactions and the impact of pathogenic mutations on SLC26A2 transporter function remain unclear. Here, using cryo-electron microscopy, we determine three high-resolution structures of SLC26A2 in complexes with different substrates. These structures unveil valuable insights, including the distinct features of the homodimer assembly, the dynamic nature of substrate binding, and the potential ramifications of pathogenic mutations. This structural-functional information regarding SLC26A2 will advance our understanding of cellular sulfate transport mechanisms and provide foundations for future therapeutic development against various human diseases. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41428.map.gz emd_41428.map.gz | 117.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41428-v30.xml emd-41428-v30.xml emd-41428.xml emd-41428.xml | 14.5 KB 14.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41428.png emd_41428.png | 145.1 KB | ||
| Filedesc metadata |  emd-41428.cif.gz emd-41428.cif.gz | 5.6 KB | ||
| Others |  emd_41428_half_map_1.map.gz emd_41428_half_map_1.map.gz emd_41428_half_map_2.map.gz emd_41428_half_map_2.map.gz | 115.7 MB 115.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41428 http://ftp.pdbj.org/pub/emdb/structures/EMD-41428 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41428 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41428 | HTTPS FTP |
-Validation report
| Summary document |  emd_41428_validation.pdf.gz emd_41428_validation.pdf.gz | 1018.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41428_full_validation.pdf.gz emd_41428_full_validation.pdf.gz | 1017.7 KB | Display | |
| Data in XML |  emd_41428_validation.xml.gz emd_41428_validation.xml.gz | 13.9 KB | Display | |
| Data in CIF |  emd_41428_validation.cif.gz emd_41428_validation.cif.gz | 16.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41428 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41428 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41428 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41428 | HTTPS FTP |
-Related structure data
| Related structure data |  8tnxMC  8tnwC  8tnyC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41428.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41428.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_41428_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_41428_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : SLC26A2 with oxalate
| Entire | Name: SLC26A2 with oxalate |
|---|---|
| Components |
|
-Supramolecule #1: SLC26A2 with oxalate
| Supramolecule | Name: SLC26A2 with oxalate / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Sulfate transporter
| Macromolecule | Name: Sulfate transporter / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 74.410359 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HRILIERQEK SDTNFKEFVI KKLQKNCQCS PAKAKNMILG FLPVLQWLPK YDLKKNILGD VMSGLIVGIL LVPQSIAYSL LAGQEPVYG LYTSFFASII YFLLGTSRHI SVGIFGVLCL MIGETVDREL QKAGYDNAHS APSLGMVSNG STLLNHTSDR I CDKSCYAI ...String: HRILIERQEK SDTNFKEFVI KKLQKNCQCS PAKAKNMILG FLPVLQWLPK YDLKKNILGD VMSGLIVGIL LVPQSIAYSL LAGQEPVYG LYTSFFASII YFLLGTSRHI SVGIFGVLCL MIGETVDREL QKAGYDNAHS APSLGMVSNG STLLNHTSDR I CDKSCYAI MVGSTVTFIA GVYQVAMGFF QVGFVSVYLS DALLSGFVTG ASFTILTSQA KYLLGLNLPR TNGVGSLITT WI HVFRNIH KTNLCDLITS LLCLLVLLPT KELNEHFKSK LKAPIPIELV VVVAATLASH FGKLHENYNS SIAGHIPTGF MPP KVPEWN LIPSVAVDAI AISIIGFAIT VSLSEMFAKK HGYTVKANQE MYAIGFCNII PSFFHCFTTS AALAKTLVKE STGC HTQLS GVVTALVLLL VLLVIAPLFY SLQKSVLGVI TIVNLRGALR KFRDLPKMWS ISRMDTVIWF VTMLSSALLS TEIGL LVGV CFSIFCVILR TQKPKSSLLG LVEESEVFES VSAYKNLQIK PGIKIFRFVA PLYYINKECF KSALYKQTVN PILIKV AWK KAAKRKIKEK VVTLGGIQDE MSVQLSHDPL ELHTIVIDCS AIQFLDTAGI HTLKEVRRDY EAIGIQVLLA QCNPTVR DS LTNGEYCKKE EENLLFYSVY EAMAFAEVSK N UniProtKB: Sulfate transporter |
-Macromolecule #2: OXALATE ION
| Macromolecule | Name: OXALATE ION / type: ligand / ID: 2 / Number of copies: 2 / Formula: OXL |
|---|---|
| Molecular weight | Theoretical: 88.019 Da |
| Chemical component information | 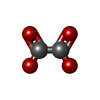 ChemComp-OXL: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.03 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 137202 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)