[English] 日本語
 Yorodumi
Yorodumi- EMDB-41344: Human Type 3 IP3 Receptor - C2 Resting TMD Transitions (Figure S8D) -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Type 3 IP3 Receptor - C2 Resting TMD Transitions (Figure S8D) | ||||||||||||
 Map data Map data | IP3 Receptor - hIP3R3 C2 Resting TMD Transitions (Figure S8D) - Consensus Refinement | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Ion Channel / Calcium Channel / Endoplasmic Reticulum / MEMBRANE PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationDAG and IP3 signaling / inositol 1,3,4,5 tetrakisphosphate binding / sensory perception of bitter taste / inositol 1,4,5-trisphosphate-gated calcium channel activity / platelet dense tubular network membrane / sensory perception of umami taste / Effects of PIP2 hydrolysis / sensory perception of sweet taste / PLC beta mediated events / Elevation of cytosolic Ca2+ levels ...DAG and IP3 signaling / inositol 1,3,4,5 tetrakisphosphate binding / sensory perception of bitter taste / inositol 1,4,5-trisphosphate-gated calcium channel activity / platelet dense tubular network membrane / sensory perception of umami taste / Effects of PIP2 hydrolysis / sensory perception of sweet taste / PLC beta mediated events / Elevation of cytosolic Ca2+ levels / inositol 1,4,5 trisphosphate binding / inositol hexakisphosphate binding / CLEC7A (Dectin-1) induces NFAT activation / transport vesicle membrane / cytoplasmic side of endoplasmic reticulum membrane / intracellularly gated calcium channel activity / brush border / nuclear outer membrane / Role of phospholipids in phagocytosis / calcium ion homeostasis / Ion homeostasis / release of sequestered calcium ion into cytosol / FCERI mediated Ca+2 mobilization / phosphatidylinositol binding / FCGR3A-mediated IL10 synthesis / Antigen activates B Cell Receptor (BCR) leading to generation of second messengers / secretory granule membrane / sarcoplasmic reticulum / VEGFR2 mediated cell proliferation / Regulation of insulin secretion / response to calcium ion / platelet activation / memory / long-term synaptic potentiation / apical part of cell / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / sensory perception of taste / positive regulation of cytosolic calcium ion concentration / Ca2+ pathway / protein homotetramerization / receptor complex / G protein-coupled receptor signaling pathway / neuronal cell body / calcium ion binding / endoplasmic reticulum membrane / nucleolus / endoplasmic reticulum / zinc ion binding / nucleoplasm / ATP binding / membrane / plasma membrane / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | ||||||||||||
 Authors Authors | Paknejad N / Sapuru V / Hite RK | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural titration reveals Ca-dependent conformational landscape of the IP receptor. Authors: Navid Paknejad / Vinay Sapuru / Richard K Hite /  Abstract: Inositol 1,4,5-trisphosphate receptors (IPRs) are endoplasmic reticulum Ca channels whose biphasic dependence on cytosolic Ca gives rise to Ca oscillations that regulate fertilization, cell division ...Inositol 1,4,5-trisphosphate receptors (IPRs) are endoplasmic reticulum Ca channels whose biphasic dependence on cytosolic Ca gives rise to Ca oscillations that regulate fertilization, cell division and cell death. Despite the critical roles of IPR-mediated Ca responses, the structural underpinnings of the biphasic Ca dependence that underlies Ca oscillations are incompletely understood. Here, we collect cryo-EM images of an IPR with Ca concentrations spanning five orders of magnitude. Unbiased image analysis reveals that Ca binding does not explicitly induce conformational changes but rather biases a complex conformational landscape consisting of resting, preactivated, activated, and inhibited states. Using particle counts as a proxy for relative conformational free energy, we demonstrate that Ca binding at a high-affinity site allows IPRs to activate by escaping a low-energy resting state through an ensemble of preactivated states. At high Ca concentrations, IPRs preferentially enter an inhibited state stabilized by a second, low-affinity Ca binding site. Together, these studies provide a mechanistic basis for the biphasic Ca-dependence of IPR channel activity. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41344.map.gz emd_41344.map.gz | 254 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41344-v30.xml emd-41344-v30.xml emd-41344.xml emd-41344.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41344.png emd_41344.png | 105.7 KB | ||
| Filedesc metadata |  emd-41344.cif.gz emd-41344.cif.gz | 7 KB | ||
| Others |  emd_41344_half_map_1.map.gz emd_41344_half_map_1.map.gz emd_41344_half_map_2.map.gz emd_41344_half_map_2.map.gz | 475.2 MB 475.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41344 http://ftp.pdbj.org/pub/emdb/structures/EMD-41344 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41344 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41344 | HTTPS FTP |
-Validation report
| Summary document |  emd_41344_validation.pdf.gz emd_41344_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41344_full_validation.pdf.gz emd_41344_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_41344_validation.xml.gz emd_41344_validation.xml.gz | 19.1 KB | Display | |
| Data in CIF |  emd_41344_validation.cif.gz emd_41344_validation.cif.gz | 22.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41344 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41344 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41344 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41344 | HTTPS FTP |
-Related structure data
| Related structure data |  8tk8C  8tkdC  8tkeC  8tkfC  8tkgC  8tkhC  8tkiC  8tl9C  8tlaC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41344.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41344.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | IP3 Receptor - hIP3R3 C2 Resting TMD Transitions (Figure S8D) - Consensus Refinement | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||||||||||||||||||
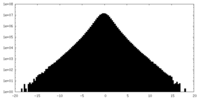
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: IP3 Receptor - hIP3R3 C2 Resting TMD Transitions...
| File | emd_41344_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | IP3 Receptor - hIP3R3 C2 Resting TMD Transitions (Figure S8D) - Consensus Refinement - Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
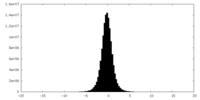
| Density Histograms |
-Half map: IP3 Receptor - hIP3R3 C2 Resting TMD Transitions...
| File | emd_41344_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | IP3 Receptor - hIP3R3 C2 Resting TMD Transitions (Figure S8D) - Consensus Refinement - Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
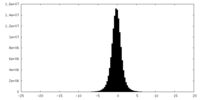
| Density Histograms |
- Sample components
Sample components
-Entire : Human Type 3 IP3 Receptor
| Entire | Name: Human Type 3 IP3 Receptor |
|---|---|
| Components |
|
-Supramolecule #1: Human Type 3 IP3 Receptor
| Supramolecule | Name: Human Type 3 IP3 Receptor / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: In the presence of saturating IP3, ATP, and Ca2+ titrated from 1 nM to 10 um |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.2 MDa |
-Macromolecule #1: Human Type 3 IP3 Receptor
| Macromolecule | Name: Human Type 3 IP3 Receptor / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSEMSSFLHI GDIVSLYAEG SVNGFISTLG LVDDRCVVEP AAGDLDNPPK KFRDCLFKVC PMNRYSAQKQ YWKAKQTKQD KEKIADVVLL QKLQHAAQME QKQNDTENKK VHGDVVKYGS VIQLLHMKSN KYLTVNKRLP ALLEKNAMRV TLDATGNEGS WLFIQPFWKL ...String: MSEMSSFLHI GDIVSLYAEG SVNGFISTLG LVDDRCVVEP AAGDLDNPPK KFRDCLFKVC PMNRYSAQKQ YWKAKQTKQD KEKIADVVLL QKLQHAAQME QKQNDTENKK VHGDVVKYGS VIQLLHMKSN KYLTVNKRLP ALLEKNAMRV TLDATGNEGS WLFIQPFWKL RSNGDNVVVG DKVILNPVNA GQPLHASNYE LSDNAGCKEV NSVNCNTSWK INLFMQFRDH LEEVLKGGDV VRLFHAEQEK FLTCDEYKGK LQVFLRTTLR QSATSATSSN ALWEVEVVHH DPCRGGAGHW NGLYRFKHLA TGNYLAAEEN PSYKGDASDP KAAGMGAQGR TGRRNAGEKI KYCLVAVPHG NDIASLFELD PTTLQKTDSF VPRNSYVRLR HLCTNTWIQS TNVPIDIEEE RPIRLMLGTC PTKEDKEAFA IVSVPVSEIR DLDFANDASS MLASAVEKLN EGFISQNDRR FVIQLLEDLV FFVSDVPNNG QNVLDIMVTK PNRERQKLMR EQNILKQVFG ILKAPFREKG GEGPLVRLEE LSDQKNAPYQ HMFRLCYRVL RHSQEDYRKN QEHIAKQFGM MQSQIGYDIL AEDTITALLH NNRKLLEKHI TKTEVETFVS LVRKNREPRF LDYLSDLCVS NHIAIPVTQE LICKCVLDPK NSDILIRTEL RPVKEMAQSH EYLSIEYSEE EVWLTWTDKN NEHHEKSVRQ LAQEARAGNA HDENVLSYYR YQLKLFARMC LDRQYLAIDE ISQQLGVDLI FLCMADEMLP FDLRASFCHL MLHVHVDRDP QELVTPVKFA RLWTEIPTAI TIKDYDSNLN ASRDDKKNKF ANTMEFVEDY LNNVVSEAVP FANEEKNKLT FEVVSLAHNL IYFGFYSFSE LLRLTRTLLG IIDCVQGPPA MLQAYEDPGG KNVRRSIQGV GHMMSTMVLS RKQSVFSAPS LSAGASAAEP LDRSKFEENE DIVVMETKLK ILEILQFILN VRLDYRISYL LSVFKKEFVE VFPMQDSGAD GTAPAFDSTT ANMNLDRIGE QAEAMFGVGK TSSMLEVDDE GGRMFLRVLI HLTMHDYAPL VSGALQLLFK HFSQRQEAMH TFKQVQLLIS AQDVENYKVI KSELDRLRTM VEKSELWVDK KGSGKGEEVE AGAAKDKKER PTDEEGFLHP PGEKSSENYQ IVKGILERLN KMCGVGEQMR KKQQRLLKNM DAHKVMLDLL QIPYDKGDAK MMEILRYTHQ FLQKFCAGNP GNQALLHKHL HLFLTPGLLE AETMQHIFLN NYQLCSEISE PVLQHFVHLL ATHGRHVQYL DFLHTVIKAE GKYVKKCQDM IMTELTNAGD DVVVFYNDKA SLAHLLDMMK AARDGVEDHS PLMYHISLVD LLAACAEGKN VYTEIKCTSL LPLEDVVSVV THEDCITEVK MAYVNFVNHC YVDTEVEMKE IYTSNHIWTL FENFTLDMAR VCSKREKRVA DPTLEKYVLS VVLDTINAFF SSPFSENSTS LQTHQTIVVQ LLQSTTRLLE CPWLQQQHKG SVEACIRTLA MVAKGRAILL PMDLDAHISS MLSSGASCAA AAQRNASSYK ATTRAFPRVT PTANQWDYKN IIEKLQDIIT ALEERLKPLV QAELSVLVDV LHWPELLFLE GSEAYQRCES GGFLSKLIQH TKDLMESEEK LCIKVLRTLQ QMLLKKTKYG DRGNQLRKML LQNYLQNRKS TSRGDLPDPI GTGLDPDWSA IAATQCRLDK EGATKLVCDL ITSTKNEKIF QESIGLAIHL LDGGNTEIQK SFHNLMMSDK KSERFFKVLH DRMKRAQQET KSTVAVNMND LGSQPHEDRE PVDPTTKGRV ASFSIPGSSS RYSLGPSLRR GHEVSERVQS SEMGTSVLIM QPILRFLQLL CENHNRDLQN FLRCQNNKTN YNLVCETLQF LDIMCGSTTG GLGLLGLYIN EDNVGLVIQT LETLTEYCQG PCHENQTCIV THESNGIDII TALILNDISP LCKYRMDLVL QLKDNASKLL LALMESRHDS ENAERILISL RPQELVDVIK KAYLQEEERE NSEVSPREVG HNIYILALQL SRHNKQLQHL LKPVKRIQEE EAEGISSMLS LNNKQLSQML KSSAPAQEEE EDPLAYYENH TSQIEIVRQD RSMEQIVFPV PGICQFLTEE TKHRLFTTTE QDEQGSKVSD FFDQSSFLHN EMEWQRKLRS MPLIYWFSRR MTLWGSISFN LAVFINIIIA FFYPYMEGAS TGVLDSPLIS LLFWILICFS IAALFTKRYS IRPLIVALIL RSIYYLGIGP TLNILGALNL TNKIVFVVSF VGNRGTFIRG YKAMVMDMEF LYHVGYILTS VLGLFAHELF YSILLFDLIY REETLFNVIK SVTRNGRSIL LTALLALILV YLFSIVGFLF LKDDFILEVD RLPNNHSTAS PLGMPHGAAA FVDTCSGDKM DCVSGLSVPE VLEEDRELDS TERACDTLLM CIVTVMNHGL RNGGGVGDIL RKPSKDESLF PARVVYDLLF FFIVIIIVLN LIFGVIIDTF ADLRSEKQKK EEILKTTCFI CGLERDKFDN KTVSFEEHIK LEHNMWNYLY FIVLVRVKNK TDYTGPESYV AQMIKNKNLD WFPRMRAMSL VSNEGEGEQN EIRILQDKLN STMKLVSHLT AQLNELKEQM TEQRKRRQRL GFVDVQNCIS R UniProtKB: Inositol 1,4,5-trisphosphate-gated calcium channel ITPR3 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 20 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 120 mM NaCl, 50 mM Tris-HCl, pH 8.0, 0.02% LMNG, 2 mM dithiothreitol, 2 mM EDTA, 2 mM EGTA, 2 mM BAPTA, 2 mM HEDTA, 1 mM ATP, 500 uM fluorinated fos-choline-8, 3 mM free Mg2+, 1-10000 nM free Ca2+ |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: Custom-made calcium free blotting paper (see publication for details).. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 6 / Number real images: 17733 / Average exposure time: 3.0 sec. / Average electron dose: 66.0 e/Å2 Details: Images were collected at 0.826 A/px magnification on an FEI Krios with Gatan K3 detector at 15 e-/pix/sec with 3 sec exposure (0.05 sec/frame) for a total dose of 66 e-/A2 in automated ...Details: Images were collected at 0.826 A/px magnification on an FEI Krios with Gatan K3 detector at 15 e-/pix/sec with 3 sec exposure (0.05 sec/frame) for a total dose of 66 e-/A2 in automated fashion using SerialEM. Five datasets were collected during the same session for each Ca2+ concentration on a series of grids that were prepared sequentially resulting in 637 movies at 1 nM, 2150 movies at 10 nM, 6126 movies at 100 nM, 1372 movies at 1 uM, and 3136 movies at 10 uM. A sixth dataset of 4312 movies collected at nominal 100 nM free Ca2+ from a grid prepared later in the sequence was collected as a technical replicate to assess experimental error. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 4.3 µm / Calibrated defocus min: 0.7000000000000001 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 63075 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: AB INITIO MODEL / Target criteria: Map to Model FSC |
|---|
 Movie
Movie Controller
Controller





















































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)