+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM of tri-pilus from S. islandicus REY15A | |||||||||
 Map data Map data | Cryo-EM map of tri-pilus | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | helical symmetry / type iv pili / archaeal pilus / filament / PROTEIN FIBRIL | |||||||||
| Function / homology | membrane / DUF973 family protein Function and homology information Function and homology information | |||||||||
| Biological species |   Sulfolobus islandicus REY15A (archaea) Sulfolobus islandicus REY15A (archaea) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.47 Å | |||||||||
 Authors Authors | Eastep GN / Liu J / Rich-New ST / Egelman EH / Krupovic M / Wang F | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Two distinct archaeal type IV pili structures formed by proteins with identical sequence. Authors: Junfeng Liu / Gunnar N Eastep / Virginija Cvirkaite-Krupovic / Shane T Rich-New / Mark A B Kreutzberger / Edward H Egelman / Mart Krupovic / Fengbin Wang /   Abstract: Type IV pili (T4P) represent one of the most common varieties of surface appendages in archaea. These filaments, assembled from small pilin proteins, can be many microns long and serve diverse ...Type IV pili (T4P) represent one of the most common varieties of surface appendages in archaea. These filaments, assembled from small pilin proteins, can be many microns long and serve diverse functions, including adhesion, biofilm formation, motility, and intercellular communication. Here, we determine atomic structures of two distinct adhesive T4P from Saccharolobus islandicus via cryo-electron microscopy (cryo-EM). Unexpectedly, both pili were assembled from the same pilin polypeptide but under different growth conditions. One filament, denoted mono-pilus, conforms to canonical archaeal T4P structures where all subunits are equivalent, whereas in the other filament, the tri-pilus, the same polypeptide exists in three different conformations. The three conformations in the tri-pilus are very different from the single conformation found in the mono-pilus, and involve different orientations of the outer immunoglobulin-like domains, mediated by a very flexible linker. Remarkably, the outer domains rotate nearly 180° between the mono- and tri-pilus conformations. Both forms of pili require the same ATPase and TadC-like membrane pore for assembly, indicating that the same secretion system can produce structurally very different filaments. Our results show that the structures of archaeal T4P appear to be less constrained and rigid than those of the homologous archaeal flagellar filaments that serve as helical propellers. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41283.map.gz emd_41283.map.gz | 9.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41283-v30.xml emd-41283-v30.xml emd-41283.xml emd-41283.xml | 16.1 KB 16.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41283.png emd_41283.png | 112.8 KB | ||
| Filedesc metadata |  emd-41283.cif.gz emd-41283.cif.gz | 5.4 KB | ||
| Others |  emd_41283_half_map_1.map.gz emd_41283_half_map_1.map.gz emd_41283_half_map_2.map.gz emd_41283_half_map_2.map.gz | 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41283 http://ftp.pdbj.org/pub/emdb/structures/EMD-41283 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41283 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41283 | HTTPS FTP |
-Related structure data
| Related structure data |  8tibMC  8tifC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41283.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41283.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM map of tri-pilus | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
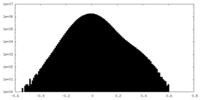
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map A
| File | emd_41283_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
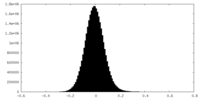
| Density Histograms |
-Half map: Half Map B
| File | emd_41283_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
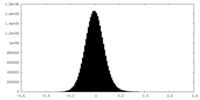
| Density Histograms |
- Sample components
Sample components
-Entire : tri-pilus
| Entire | Name: tri-pilus |
|---|---|
| Components |
|
-Supramolecule #1: tri-pilus
| Supramolecule | Name: tri-pilus / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Sulfolobus islandicus REY15A (archaea) Sulfolobus islandicus REY15A (archaea) |
-Macromolecule #1: DUF973 family protein
| Macromolecule | Name: DUF973 family protein / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Sulfolobus islandicus REY15A (archaea) Sulfolobus islandicus REY15A (archaea) |
| Molecular weight | Theoretical: 14.053037 KDa |
| Sequence | String: MNMISRRKNA KALSGAVTAL ILVIASVIIA LVVVGFAFGL FGAFTGQGTV TQVGTATLSA GTGTLTVTLK NTGAATQVTG AIINGNAAS VSGQVTISAG QNTYSISLGG ISSSTLQNLV GSTISLTLQL SNGQTVTVSA IITS UniProtKB: DUF973 family protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 15.8 Å Applied symmetry - Helical parameters - Δ&Phi: -39.65 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.47 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 240930 |
|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| Startup model | Type of model: NONE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)