[English] 日本語
 Yorodumi
Yorodumi- EMDB-41278: Cryo-EM structure of a SUR1/Kir6.2-Q52R ATP-sensitive potassium c... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of a SUR1/Kir6.2-Q52R ATP-sensitive potassium channel in the presence of PIP2 in the open conformation | |||||||||
 Map data Map data | full unsharpened, unmodified, original map from cryoSPARC C4 non-uniform refinement | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATP-sensitive potassium channel / KATP channel / SUR1 / Kir6.2-Q52R / potassium transport / metabolic sensor / diabetes / phospholipid binding / PIP2 / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationRegulation of insulin secretion / ATP sensitive Potassium channels / ABC-family proteins mediated transport / ATP-activated inward rectifier potassium channel activity / response to resveratrol / inward rectifying potassium channel / sulfonylurea receptor activity / ventricular cardiac muscle tissue development / cell body fiber / CAMKK-AMPK signaling cascade ...Regulation of insulin secretion / ATP sensitive Potassium channels / ABC-family proteins mediated transport / ATP-activated inward rectifier potassium channel activity / response to resveratrol / inward rectifying potassium channel / sulfonylurea receptor activity / ventricular cardiac muscle tissue development / cell body fiber / CAMKK-AMPK signaling cascade / regulation of monoatomic ion transmembrane transport / voltage-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / ATPase-coupled monoatomic cation transmembrane transporter activity / inward rectifier potassium channel activity / nervous system process / : / ankyrin binding / Ion homeostasis / response to testosterone / response to ATP / potassium ion import across plasma membrane / response to stress / action potential / intercalated disc / voltage-gated potassium channel activity / axolemma / ABC-type transporter activity / cellular response to nutrient levels / heat shock protein binding / potassium ion transmembrane transport / T-tubule / regulation of insulin secretion / acrosomal vesicle / response to ischemia / determination of adult lifespan / regulation of membrane potential / positive regulation of protein localization to plasma membrane / cellular response to glucose stimulus / negative regulation of insulin secretion / sarcolemma / potassium ion transport / cellular response to nicotine / glucose metabolic process / cellular response to tumor necrosis factor / nuclear envelope / response to estradiol / presynaptic membrane / transmembrane transporter binding / response to hypoxia / endosome / response to xenobiotic stimulus / neuronal cell body / apoptotic process / glutamatergic synapse / ATP hydrolysis activity / ATP binding / metal ion binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Mesocricetus auratus (golden hamster) Mesocricetus auratus (golden hamster) | |||||||||
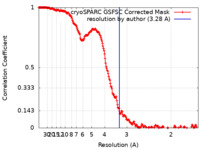
| Method | single particle reconstruction / cryo EM / Resolution: 3.28 Å | |||||||||
 Authors Authors | Driggers CM / Shyng S-L | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structure of an open K channel reveals tandem PIP binding sites mediating the Kir6.2 and SUR1 regulatory interface. Authors: Camden M Driggers / Yi-Ying Kuo / Phillip Zhu / Assmaa ElSheikh / Show-Ling Shyng /   Abstract: ATP-sensitive potassium (K) channels, composed of four pore-lining Kir6.2 subunits and four regulatory sulfonylurea receptor 1 (SUR1) subunits, control insulin secretion in pancreatic β-cells. K ...ATP-sensitive potassium (K) channels, composed of four pore-lining Kir6.2 subunits and four regulatory sulfonylurea receptor 1 (SUR1) subunits, control insulin secretion in pancreatic β-cells. K channel opening is stimulated by PIP and inhibited by ATP. Mutations that increase channel opening by PIP reduce ATP inhibition and cause neonatal diabetes. Although considerable evidence has implicated a role for PIP in K channel function, previously solved open-channel structures have lacked bound PIP, and mechanisms by which PIP regulates K channels remain unresolved. Here, we report the cryoEM structure of a K channel harboring the neonatal diabetes mutation Kir6.2-Q52R, in the open conformation, bound to amphipathic molecules consistent with natural C18:0/C20:4 long-chain PI(4,5)P at two adjacent binding sites between SUR1 and Kir6.2. The canonical PIP binding site is conserved among PIP-gated Kir channels. The non-canonical PIP binding site forms at the interface of Kir6.2 and SUR1. Functional studies demonstrate both binding sites determine channel activity. Kir6.2 pore opening is associated with a twist of the Kir6.2 cytoplasmic domain and a rotation of the N-terminal transmembrane domain of SUR1, which widens the inhibitory ATP binding pocket to disfavor ATP binding. The open conformation is particularly stabilized by the Kir6.2-Q52R residue through cation-π bonding with SUR1-W51. Together, these results uncover the cooperation between SUR1 and Kir6.2 in PIP binding and gating, explain the antagonistic regulation of K channels by PIP and ATP, and provide a putative mechanism by which Kir6.2-Q52R stabilizes an open channel to cause neonatal diabetes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41278.map.gz emd_41278.map.gz | 410.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41278-v30.xml emd-41278-v30.xml emd-41278.xml emd-41278.xml | 24.9 KB 24.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41278_fsc.xml emd_41278_fsc.xml | 22.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_41278.png emd_41278.png | 131.3 KB | ||
| Masks |  emd_41278_msk_1.map emd_41278_msk_1.map | 824 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-41278.cif.gz emd-41278.cif.gz | 8.5 KB | ||
| Others |  emd_41278_half_map_1.map.gz emd_41278_half_map_1.map.gz emd_41278_half_map_2.map.gz emd_41278_half_map_2.map.gz | 763.9 MB 764 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41278 http://ftp.pdbj.org/pub/emdb/structures/EMD-41278 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41278 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41278 | HTTPS FTP |
-Validation report
| Summary document |  emd_41278_validation.pdf.gz emd_41278_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41278_full_validation.pdf.gz emd_41278_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_41278_validation.xml.gz emd_41278_validation.xml.gz | 28.6 KB | Display | |
| Data in CIF |  emd_41278_validation.cif.gz emd_41278_validation.cif.gz | 37.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41278 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41278 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41278 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41278 | HTTPS FTP |
-Related structure data
| Related structure data |  8ti2MC  8ti1C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41278.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41278.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | full unsharpened, unmodified, original map from cryoSPARC C4 non-uniform refinement | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_41278_msk_1.map emd_41278_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map A from cryoSPARC C4 non-uniform refinement
| File | emd_41278_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A from cryoSPARC C4 non-uniform refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map B from cryoSPARC C4 non-uniform refinement
| File | emd_41278_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B from cryoSPARC C4 non-uniform refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Kir6.2-Q52R/SUR1 open channel
| Entire | Name: Kir6.2-Q52R/SUR1 open channel |
|---|---|
| Components |
|
-Supramolecule #1: Kir6.2-Q52R/SUR1 open channel
| Supramolecule | Name: Kir6.2-Q52R/SUR1 open channel / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 Details: Kir6.2-Q52R/SUR1 open KATP channel in the open conformation in complex with PIP2 and other phospholipids |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 880 KDa |
-Macromolecule #1: ATP-sensitive inward rectifier potassium channel 11
| Macromolecule | Name: ATP-sensitive inward rectifier potassium channel 11 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 43.690828 KDa |
| Recombinant expression | Organism:  Chlorocebus aethiops (grivet) Chlorocebus aethiops (grivet) |
| Sequence | String: MLSRKGIIPE EYVLTRLAED PTEPRYRTRE RRARFVSKKG NCNVAHKNIR ERGRFLQDVF TTLVDLKWPH TLLIFTMSFL CSWLLFAMV WWLIAFAHGD LAPGEGTNVP CVTSIHSFSS AFLFSIEVQV TIGFGGRMVT EECPLAILIL IVQNIVGLMI N AIMLGCIF ...String: MLSRKGIIPE EYVLTRLAED PTEPRYRTRE RRARFVSKKG NCNVAHKNIR ERGRFLQDVF TTLVDLKWPH TLLIFTMSFL CSWLLFAMV WWLIAFAHGD LAPGEGTNVP CVTSIHSFSS AFLFSIEVQV TIGFGGRMVT EECPLAILIL IVQNIVGLMI N AIMLGCIF MKTAQAHRRA ETLIFSKHAV ITLRHGRLCF MLRVGDLRKS MIISATIHMQ VVRKTTSPEG EVVPLHQVDI PM ENGVGGN SIFLVAPLII YHVIDSNSPL YDLAPSDLHH HQDLEIIVIL EGVVETTGIT TQARTSYLAD EILWGQRFVP IVA EEDGRY SVDYSKFGNT VKVPTPLCTA RQLDEDRSLL DALTLASSRG PLRKRSVAVA KAKPKFSISP DSLS UniProtKB: ATP-sensitive inward rectifier potassium channel 11 |
-Macromolecule #2: SUR1
| Macromolecule | Name: SUR1 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mesocricetus auratus (golden hamster) Mesocricetus auratus (golden hamster) |
| Molecular weight | Theoretical: 177.296578 KDa |
| Recombinant expression | Organism:  Chlorocebus aethiops (grivet) Chlorocebus aethiops (grivet) |
| Sequence | String: MPLAFCGTEN HSAAYRVDQG VLNNGCFVDA LNVVPHVFLL FITFPILFIG WGSQSSKVHI HHSTWLHFPG HNLRWILTFI LLFVLVCEI AEGILSDGVT ESRHLHLYMP AGMAFMAAIT SVVYYHNIET SNFPKLLIAL LIYWTLAFIT KTIKFVKFYD H AIGFSQLR ...String: MPLAFCGTEN HSAAYRVDQG VLNNGCFVDA LNVVPHVFLL FITFPILFIG WGSQSSKVHI HHSTWLHFPG HNLRWILTFI LLFVLVCEI AEGILSDGVT ESRHLHLYMP AGMAFMAAIT SVVYYHNIET SNFPKLLIAL LIYWTLAFIT KTIKFVKFYD H AIGFSQLR FCLTGLLVIL YGMLLLVEVN VIRVRRYIFF KTPREVKPPE DLQDLGVRFL QPFVNLLSKG TYWWMNAFIK TA HKKPIDL RAIGKLPIAM RALTNYQRLC VAFDAQARKD TQSPQGARAI WRALCHAFGR RLILSSTFRI LADLLGFAGP LCI FGIVDH LGKENHVFQP KTQFLGVYFV SSQEFLGNAY VLAVLLFLAL LLQRTFLQAS YYVAIETGIN LRGAIQTKIY NKIM HLSTS NLSMGEMTAG QICNLVAIDT NQLMWFFFLC PNLWAMPVQI IVGVILLYYI LGVSALIGAA VIILLAPVQY FVATK LSQA QRSTLEHSNE RLKQTNEMLR GMKLLKLYAW ESIFCSRVEV TRRKEMTSLR AFAVYTSISI FMNTAIPIAA VLITFV GHV SFFKESDLSP SVAFASLSLF HILVTPLFLL SSVVRSTVKA LVSVKKLSEF LSSAEIREEQ CAPREPAPQG QAGKYQA VP LKVVNRKRPA REEVRDLLGP LQRLAPSMDG DADNFCVQII GGFFTWTPDG IPTLSNITIR IPRGQLTMIV GQVGCGKS S LLLATLGEMQ KVSGAVFWNS NLPDSEGEDP SSPERETAAG SDIRSRGPVA YASQKPWLLN ATVEENITFE SPFNKQRYK MVIEACSLQP DIDILPHGDQ TQIGERGINL SGGQRQRISV ARALYQQTNV VFLDDPFSAL DVHLSDHLMQ AGILELLRDD KRTVVLVTH KLQYLPHADW IIAMKDGTIQ REGTLKDFQR SECQLFEHWK TLMNRQDQEL EKETVMERKA SEPSQGLPRA M SSRDGLLL DEEEEEEEAA ESEEDDNLSS VLHQRAKIPW RACTKYLSSA GILLLSLLVF SQLLKHMVLV AIDYWLAKWT DS ALVLSPA ARNCSLSQEC DLDQSVYAMV FTLLCSLGIV LCLVTSVTVE WTGLKVAKRL HRSLLNRIIL APMRFFETTP LGS ILNRFS SDCNTIDQHI PSTLECLSRS TLLCVSALTV ISYVTPVFLV ALLPLAVVCY FIQKYFRVAS RDLQQLDDTT QLPL LSHFA ETVEGLTTIR AFRYEARFQQ KLLEYTDSNN IASLFLTAAN RWLEVRMEYI GACVVLIAAA TSISNSLHRE LSAGL VGLG LTYALMVSNY LNWMVRNLAD MEIQLGAVKR IHALLKTEAE SYEGLLAPSL IPKNWPDQGK IQIQNLSVRY DSSLKP VLK HVNALISPGQ KIGICGRTGS GKSSFSLAFF RMVDMFEGRI IIDGIDIAKL PLHTLRSRLS IILQDPVLFS GTIRFNL DP EKKCSDSTLW EALEIAQLKL VVKALPGGLD AIITEGGENF SQGQRQLFCL ARAFVRKTSI FIMDEATASI DMATENIL Q KVVMTAFADR TVVTIAHRVH TILSADLVMV LKRGAILEFD KPETLLSQKD SVFASFVRAD K UniProtKB: ATP-binding cassette sub-family C member 8 |
-Macromolecule #3: [(2R)-1-octadecanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tr...
| Macromolecule | Name: [(2R)-1-octadecanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tris(oxidanyl)-4,5-diphosphonooxy-cyclohexyl]oxy-phospho ryl]oxy-propan-2-yl] (8Z)-icosa-5,8,11,14-tetraenoate type: ligand / ID: 3 / Number of copies: 8 / Formula: PT5 |
|---|---|
| Molecular weight | Theoretical: 1.047088 KDa |
-Macromolecule #4: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 4 / Number of copies: 4 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Macromolecule #5: DI-PALMITOYL-3-SN-PHOSPHATIDYLETHANOLAMINE
| Macromolecule | Name: DI-PALMITOYL-3-SN-PHOSPHATIDYLETHANOLAMINE / type: ligand / ID: 5 / Number of copies: 4 / Formula: PEF |
|---|---|
| Molecular weight | Theoretical: 691.959 Da |
| Chemical component information |  ChemComp-PEF: |
-Macromolecule #6: O-[(R)-{[(2R)-2,3-bis(octadecanoyloxy)propyl]oxy}(hydroxy)phospho...
| Macromolecule | Name: O-[(R)-{[(2R)-2,3-bis(octadecanoyloxy)propyl]oxy}(hydroxy)phosphoryl]-L-serine type: ligand / ID: 6 / Number of copies: 8 / Formula: P5S |
|---|---|
| Molecular weight | Theoretical: 792.075 Da |
| Chemical component information |  ChemComp-P5S: |
-Macromolecule #7: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 7 / Number of copies: 4 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #8: water
| Macromolecule | Name: water / type: ligand / ID: 8 / Number of copies: 14 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: MSB with digitonin and PIP2 | ||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE OXIDE / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. Details: The grid was prepared with a Graphene Oxide coating before use. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 279 K / Instrument: FEI VITROBOT MARK III | ||||||||||||||||||
| Details | 3 microliters of purified Kir6.2-Q52R/FLAG-SUR1 was loaded onto Quantifoil R 1.2/1.3 Au 300 grids prepared with a fresh Graphene Oxide surface |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Details | Titan Krios #3 at the Pacific Northwest National Lab |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 3 / Number real images: 5241 / Average exposure time: 2.2 sec. / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)