+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of AtMSL10-K539E | |||||||||
 Map data Map data | NU-refinement | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channels / mechanosensitive channels / heptamer / Arabidopsis thaliana / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationprogrammed cell death in response to reactive oxygen species / leaf senescence / detection of mechanical stimulus / mechanosensitive monoatomic ion channel activity / monoatomic anion transport / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Zhang J / Yuan P | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Open structure and gating of the Arabidopsis mechanosensitive ion channel MSL10. Authors: Jingying Zhang / Grigory Maksaev / Peng Yuan /  Abstract: Plants are challenged by drastically different osmotic environments during growth and development. Adaptation to these environments often involves mechanosensitive ion channels that can detect and ...Plants are challenged by drastically different osmotic environments during growth and development. Adaptation to these environments often involves mechanosensitive ion channels that can detect and respond to mechanical force. In the model plant Arabidopsis thaliana, the mechanosensitive channel MSL10 plays a crucial role in hypo-osmotic shock adaptation and programmed cell death induction, but the molecular basis of channel function remains poorly understood. Here, we report a structural and electrophysiological analysis of MSL10. The cryo-electron microscopy structures reveal a distinct heptameric channel assembly. Structures of the wild-type channel in detergent and lipid environments, and in the absence of membrane tension, capture an open conformation. Furthermore, structural analysis of a non-conductive mutant channel demonstrates that reorientation of phenylalanine side chains alone, without main chain rearrangements, may generate the hydrophobic gate. Together, these results reveal a distinct gating mechanism and advance our understanding of mechanotransduction. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41168.map.gz emd_41168.map.gz | 44.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41168-v30.xml emd-41168-v30.xml emd-41168.xml emd-41168.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41168.png emd_41168.png | 141.2 KB | ||
| Filedesc metadata |  emd-41168.cif.gz emd-41168.cif.gz | 6.4 KB | ||
| Others |  emd_41168_additional_1.map.gz emd_41168_additional_1.map.gz emd_41168_additional_2.map.gz emd_41168_additional_2.map.gz emd_41168_half_map_1.map.gz emd_41168_half_map_1.map.gz emd_41168_half_map_2.map.gz emd_41168_half_map_2.map.gz | 78.4 MB 85.7 MB 84.1 MB 84.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41168 http://ftp.pdbj.org/pub/emdb/structures/EMD-41168 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41168 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41168 | HTTPS FTP |
-Related structure data
| Related structure data |  8tdmMC  8tdjC  8tdkC  8tdlC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41168.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41168.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | NU-refinement | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.2 Å | ||||||||||||||||||||||||||||||||||||
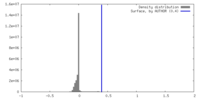
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: deepEMhancer
| File | emd_41168_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | deepEMhancer | ||||||||||||
| Projections & Slices |
| ||||||||||||
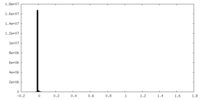
| Density Histograms |
-Additional map: #1
| File | emd_41168_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
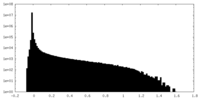
| Density Histograms |
-Half map: half B
| File | emd_41168_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_B | ||||||||||||
| Projections & Slices |
| ||||||||||||
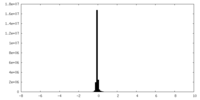
| Density Histograms |
-Half map: half A
| File | emd_41168_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : AtMSL10 with mutation K539E
| Entire | Name: AtMSL10 with mutation K539E |
|---|---|
| Components |
|
-Supramolecule #1: AtMSL10 with mutation K539E
| Supramolecule | Name: AtMSL10 with mutation K539E / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Mechanosensitive ion channel protein 10
| Macromolecule | Name: Mechanosensitive ion channel protein 10 / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 83.9555 KDa |
| Recombinant expression | Organism:  Komagataella pastoris (fungus) Komagataella pastoris (fungus) |
| Sequence | String: MAEQKSSNGG GGGGDVVINV PVEEASRRSK EMASPESEKG VPFSKSPSPE ISKLVGSPNK PPRAPNQNNV GLTQRKSFAR SVYSKPKSR FVDPSCPVDT SILEEEVREQ LGAGFSFSRA SPNNKSNRSV GSPAPVTPSK VVVEKDEDEE IYKKVKLNRE M RSKISTLA ...String: MAEQKSSNGG GGGGDVVINV PVEEASRRSK EMASPESEKG VPFSKSPSPE ISKLVGSPNK PPRAPNQNNV GLTQRKSFAR SVYSKPKSR FVDPSCPVDT SILEEEVREQ LGAGFSFSRA SPNNKSNRSV GSPAPVTPSK VVVEKDEDEE IYKKVKLNRE M RSKISTLA LIESAFFVVI LSALVASLTI NVLKHHTFWG LEVWKWCVLV MVIFSGMLVT NWFMRLIVFL IETNFLLRRK VL YFVHGLK KSVQVFIWLC LILVAWILLF NHDVKRSPAA TKVLKCITRT LISILTGAFF WLVKTLLLKI LAANFNVNNF FDR IQDSVF HQYVLQTLSG LPLMEEAERV GREPSTGHLS FATVVKKGTV KEKKVIDMGK VHKMKREKVS AWTMRVLMEA VRTS GLSTI SDTLDETAYG EGKEQADREI TSEMEALAAA YHVFRNVAQP FFNYIEEEDL LRFMIKEEVD LVFPLFDGAA ETGRI TRKA FTEWVVKVYT SRRALAHSLN DTKTAVKQLN KLVTAILMVV TVVIWLLLLE VATTEVLLFF STQLVALAFI IGSTCK NLF ESIVFVFVMH PYDVGDRCVV DGVAMLVEEM NLLTTVFLKL NNEKVYYPNA VLATKPISNY FRSPNMGETV EFSISFS TP VSKIAHLKER IAEYLEQNPQ HWAPVHSVVV KEIENMNKLK MALYSDHTIT FQENRERNLR RTELSLAIKR MLEDLHID Y TLLPQDINLT KKNSLEVLFQ UniProtKB: Mechanosensitive ion channel protein 10 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
Details: 20 mM Tris-HCl PH 8.0, 150 mM NaCl, and 0.04 mM GDN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||
| Details | AtMSL10-K539E in detergent |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number real images: 3229 / Average electron dose: 44.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 120000 |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)