+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | PS3 F1 Rotorless, high ATP | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATPase / Membrane protein / electron transport / TRANSLOCASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationH+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / ADP binding / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.95 Å | |||||||||
 Authors Authors | Sobti M / Stewart AG | |||||||||
| Funding support |  Australia, 1 items Australia, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: The series of conformational states adopted by rotorless F-ATPase during its hydrolysis cycle. Authors: Meghna Sobti / Hiroshi Ueno / Simon H J Brown / Hiroyuki Noji / Alastair G Stewart /   Abstract: FF ATP synthase interchanges phosphate transfer energy and proton motive force via a rotary catalytic mechanism and isolated F-ATPase subcomplexes can also hydrolyze ATP to generate rotation of their ...FF ATP synthase interchanges phosphate transfer energy and proton motive force via a rotary catalytic mechanism and isolated F-ATPase subcomplexes can also hydrolyze ATP to generate rotation of their central γ rotor subunit. As ATP is hydrolyzed, the F-ATPase cycles through a series of conformational states that mediates unidirectional rotation of the rotor. However, even in the absence of a rotor, the α and β subunits are still able to pass through a series of conformations, akin to those that generate rotation. Here, we use cryoelectron microscopy to establish the structures of these rotorless states. These structures indicate that cooperativity in this system is likely mediated by contacts between the β subunit lever domains, irrespective of the presence of the γ rotor subunit. These findings provide insight into how long-range information may be transferred in large biological systems. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40689.map.gz emd_40689.map.gz | 59.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40689-v30.xml emd-40689-v30.xml emd-40689.xml emd-40689.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_40689.png emd_40689.png | 132.1 KB | ||
| Filedesc metadata |  emd-40689.cif.gz emd-40689.cif.gz | 6 KB | ||
| Others |  emd_40689_additional_1.map.gz emd_40689_additional_1.map.gz emd_40689_half_map_1.map.gz emd_40689_half_map_1.map.gz emd_40689_half_map_2.map.gz emd_40689_half_map_2.map.gz | 54.5 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40689 http://ftp.pdbj.org/pub/emdb/structures/EMD-40689 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40689 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40689 | HTTPS FTP |
-Validation report
| Summary document |  emd_40689_validation.pdf.gz emd_40689_validation.pdf.gz | 1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_40689_full_validation.pdf.gz emd_40689_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  emd_40689_validation.xml.gz emd_40689_validation.xml.gz | 12.2 KB | Display | |
| Data in CIF |  emd_40689_validation.cif.gz emd_40689_validation.cif.gz | 14.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40689 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40689 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40689 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40689 | HTTPS FTP |
-Related structure data
| Related structure data |  8spxMC  8spvC  8spwC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40689.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40689.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
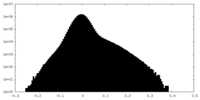
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_40689_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_40689_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_40689_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : PS3 F1 Rotorless
| Entire | Name: PS3 F1 Rotorless |
|---|---|
| Components |
|
-Supramolecule #1: PS3 F1 Rotorless
| Supramolecule | Name: PS3 F1 Rotorless / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: ATP synthase subunit alpha
| Macromolecule | Name: ATP synthase subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO / EC number: H+-transporting two-sector ATPase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 51.875262 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SDVGTVIQVG DGIARAHGLD NVMSGELVEF ANGVMGMALN LEENNVGIVI LGPYTGIKEG DEVRRTGRIM EVPVGEALIG RVVNPLGQP VDGLGPVETT ETRPIESRAP GVMDRRSVHE PLQTGIKAID ALVPIGRGQR ELIIGDRQTG KTSVAIDTII N QKDQNMIS ...String: SDVGTVIQVG DGIARAHGLD NVMSGELVEF ANGVMGMALN LEENNVGIVI LGPYTGIKEG DEVRRTGRIM EVPVGEALIG RVVNPLGQP VDGLGPVETT ETRPIESRAP GVMDRRSVHE PLQTGIKAID ALVPIGRGQR ELIIGDRQTG KTSVAIDTII N QKDQNMIS IYVAIGQKES TVRTVVETLR KHGALDYTIV VTASASQPAP LLFLAPYAGV AMGEYFMYKG KHVLVVYDDL SK QAAAYRE LSLLLRRPPG REAYPGDIFY LHSRLLERAA KLSDAKGGGS LTALPFVETQ AGDISAYIPT NVISITDGQI FLQ SDLFFS GVRPAINAGL SVSRVGGAAQ IKAMKKVAGT LRLDLAAYRE LEAFAQFGSD LDKATQAKLA RGARTVEVLK QDLH QPIPV EKQVLIIYAL TRGFLDDIPV EDVRRFEKEF YLFLDQNGQH LLEHIRTTKD LPNEDDLNKA IEAFKKTFVV S UniProtKB: ATP synthase subunit alpha |
-Macromolecule #2: ATP synthase subunit beta
| Macromolecule | Name: ATP synthase subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 51.683727 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTRGRVIQVM GPVVDVKFEN GHLPAIYNAL KIQHKARNEN EVDIDLTLEV ALHLGDDTVR TIAMASTDGL IRGMEVIDTG APISVPVGE VTLGRVFNVL GEPIDLEGDI PADARRDPIH RPAPKFEELA TEVEILETGI KVVDLLAPYI KGGKIGLFGG A GVGKTVLI ...String: MTRGRVIQVM GPVVDVKFEN GHLPAIYNAL KIQHKARNEN EVDIDLTLEV ALHLGDDTVR TIAMASTDGL IRGMEVIDTG APISVPVGE VTLGRVFNVL GEPIDLEGDI PADARRDPIH RPAPKFEELA TEVEILETGI KVVDLLAPYI KGGKIGLFGG A GVGKTVLI QELIHNIAQE HGGISVFAGV GERTREGNDL YHEMKDSGVI SKTAMVFGQM NEPPGARMRV ALTGLTMAEY FR DEQGQDV LLFIDNIFRF TQAGSEVSAL LGRMPSAVGY QPTLATEMGQ LQERITSTAK GSITSIQAIY VPADDYTDPA PAT TFSHLD ATTNLERKLA EMGIYPAVDP LASTSRALAP EIVGEEHYQV ARKVQQTLQR YKELQDIIAI LGMDELSDED KLVV HRARR IQFFLSQNFH VAEQFTGQPG SYVPVKETVR GFKEILEGKY DHLPEDAFRL VGRIEEVVEK AKAMGV UniProtKB: ATP synthase subunit beta |
-Macromolecule #3: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 5 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 6 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #6: PHOSPHATE ION
| Macromolecule | Name: PHOSPHATE ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: PO4 |
|---|---|
| Molecular weight | Theoretical: 94.971 Da |
| Chemical component information |  ChemComp-PO4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.95 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 218396 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)