+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human ULK1 complex core (2:1:1 stoichiometry) | |||||||||
 Map data Map data | Composite map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Autophagy / Protein kinase / Complex / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationneuron projection regeneration / omegasome membrane / negative regulation of collateral sprouting / Atg1/ULK1 kinase complex / ribophagy / response to mitochondrial depolarisation / glycophagy / positive regulation of autophagosome assembly / protein localization to phagophore assembly site / phagophore assembly site membrane ...neuron projection regeneration / omegasome membrane / negative regulation of collateral sprouting / Atg1/ULK1 kinase complex / ribophagy / response to mitochondrial depolarisation / glycophagy / positive regulation of autophagosome assembly / protein localization to phagophore assembly site / phagophore assembly site membrane / autophagy of mitochondrion / piecemeal microautophagy of the nucleus / pexophagy / RAB GEFs exchange GTP for GDP on RABs / protein kinase regulator activity / regulation of tumor necrosis factor-mediated signaling pathway / phagophore assembly site / axon extension / TBC/RABGAPs / reticulophagy / : / response to starvation / cellular response to stress / Macroautophagy / Receptor Mediated Mitophagy / autophagosome membrane / positive regulation of cell size / autophagosome assembly / regulation of macroautophagy / cellular response to nutrient levels / negative regulation of protein-containing complex assembly / mitophagy / extrinsic apoptotic signaling pathway / protein-membrane adaptor activity / positive regulation of autophagy / autophagosome / protein serine/threonine kinase activator activity / negative regulation of extrinsic apoptotic signaling pathway / macroautophagy / liver development / Regulation of TNFR1 signaling / peptidyl-serine phosphorylation / recycling endosome / positive regulation of JNK cascade / autophagy / small GTPase binding / regulation of autophagy / neuron projection development / intracellular protein localization / protein autophosphorylation / heart development / GTPase binding / nuclear membrane / defense response to virus / molecular adaptor activity / mitochondrial outer membrane / protein phosphorylation / lysosome / non-specific serine/threonine protein kinase / axon / negative regulation of cell population proliferation / innate immune response / protein serine kinase activity / protein serine/threonine kinase activity / protein kinase binding / endoplasmic reticulum membrane / protein-containing complex binding / signal transduction / mitochondrion / ATP binding / identical protein binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Chen M / Hurley JH | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2023 Journal: bioRxiv / Year: 2023Title: Structure and activation of the human autophagy-initiating ULK1C:PI3KC3-C1 supercomplex Authors: Chen M / Ren X / Cook A / Hurley JH | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40658.map.gz emd_40658.map.gz | 166.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40658-v30.xml emd-40658-v30.xml emd-40658.xml emd-40658.xml | 25.8 KB 25.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_40658.png emd_40658.png | 49.5 KB | ||
| Filedesc metadata |  emd-40658.cif.gz emd-40658.cif.gz | 6.8 KB | ||
| Others |  emd_40658_additional_1.map.gz emd_40658_additional_1.map.gz emd_40658_additional_2.map.gz emd_40658_additional_2.map.gz emd_40658_additional_3.map.gz emd_40658_additional_3.map.gz emd_40658_additional_4.map.gz emd_40658_additional_4.map.gz | 167.9 MB 166.1 MB 165.3 MB 165.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40658 http://ftp.pdbj.org/pub/emdb/structures/EMD-40658 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40658 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40658 | HTTPS FTP |
-Related structure data
| Related structure data |  8soiMC  8sorC  8sqzC  8srmC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40658.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40658.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||
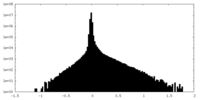
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Consensus map
| File | emd_40658_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Consensus map | ||||||||||||
| Projections & Slices |
| ||||||||||||
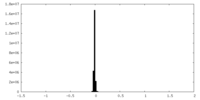
| Density Histograms |
-Additional map: Local refinement map 2/3
| File | emd_40658_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement map 2/3 | ||||||||||||
| Projections & Slices |
| ||||||||||||
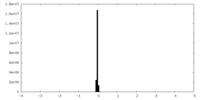
| Density Histograms |
-Additional map: Local refinement map 1/3
| File | emd_40658_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement map 1/3 | ||||||||||||
| Projections & Slices |
| ||||||||||||
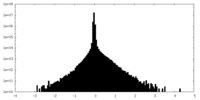
| Density Histograms |
-Additional map: Local refinement map 3/3
| File | emd_40658_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement map 3/3 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human autophagy initiation ULK1 complex core
| Entire | Name: Human autophagy initiation ULK1 complex core |
|---|---|
| Components |
|
-Supramolecule #1: Human autophagy initiation ULK1 complex core
| Supramolecule | Name: Human autophagy initiation ULK1 complex core / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 188 KDa |
-Macromolecule #1: RB1-inducible coiled-coil protein 1
| Macromolecule | Name: RB1-inducible coiled-coil protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 68.808477 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKLYVFLVNT GTTLTFDTEL TVQTVADLKH AIQSKYKIAI QHQVLVVNGG ECMAADRRVC TYSAGTDTNP IFLFNKEMIL CDRPPAIPK TTFSTENDME IKVEESLMMP AVFHTVASRT QLALEMYEVA KKLCSFCEGL VHDEHLQHQG WAAIMANLED C SNSYQKLL ...String: MKLYVFLVNT GTTLTFDTEL TVQTVADLKH AIQSKYKIAI QHQVLVVNGG ECMAADRRVC TYSAGTDTNP IFLFNKEMIL CDRPPAIPK TTFSTENDME IKVEESLMMP AVFHTVASRT QLALEMYEVA KKLCSFCEGL VHDEHLQHQG WAAIMANLED C SNSYQKLL FKFESIYSNY LQSIEDIKLK LTHLGTAVSV MAKIPLLECL TRHSYRECLG RLDSLPEHED SEKAEMKRST EL VLSPDMP RTTNESLLTS FPKSVEHVSP DTADAESGKE IRESCQSTVH QQDETTIDTK DGDLPFFNVS LLDWINVQDR PND VESLVR KCFDSMSRLD PRIIRPFIAE CRQTIAKLDN QNMKAIKGLE DRLYALDQMI ASCGRLVNEQ KELAQGFLAN QKRA ENLKD ASVLPDLCLS HANQLMIMLQ NHRKLLDIKQ KCTTAKQELA NNLHVRLKWC CFVMLHADQD GEKLQALLRL VIELL ERVK IVEALSTVPQ MYCLAVVEVV RRKMFIKHYR EWAGALVKDG KRLYEAEKSK RESFGKLFRK SFLRNRLFRG LDSWPP SFC TQKPRKFDCE LPDISLKDLQ FLQSFCPSEV QPFLRVP UniProtKB: RB1-inducible coiled-coil protein 1 |
-Macromolecule #2: Serine/threonine-protein kinase ULK1
| Macromolecule | Name: Serine/threonine-protein kinase ULK1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.113896 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: HTEILRGLRF TLLFVQHVLE IAALKGSASE AAGGPEYQLQ ESVVADQISL LSREWGFAEQ LVLYLKVAEL LSSGLQSAID QIRAGKLCL SSTVKQVVRR LNELYKASVV SCQGLSLRLQ RFFLDKQRLL DRIHSITAER LIFSHAVQMV QSAALDEMFQ H REGCVPRY ...String: HTEILRGLRF TLLFVQHVLE IAALKGSASE AAGGPEYQLQ ESVVADQISL LSREWGFAEQ LVLYLKVAEL LSSGLQSAID QIRAGKLCL SSTVKQVVRR LNELYKASVV SCQGLSLRLQ RFFLDKQRLL DRIHSITAER LIFSHAVQMV QSAALDEMFQ H REGCVPRY HKALLLLEGL QHMLSDQADI ENVTKCKLCI ERRLSAL UniProtKB: Serine/threonine-protein kinase ULK1 |
-Macromolecule #3: Autophagy-related protein 13
| Macromolecule | Name: Autophagy-related protein 13 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.805392 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DLGTFYREFQ NPPQLSSLSI DIGAQSMAED LDSLPEKLAV HEKNVREFDA FVETLQGSDE A UniProtKB: Autophagy-related protein 13 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||
| Grid | Model: UltrAuFoil / Material: GOLD / Mesh: 300 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: GOLD / Support film - #0 - topology: HOLEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: GRAPHENE / Support film - #1 - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: AIR / Details: 5 mA | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 8468 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
| Output model |  PDB-8soi: |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)