[English] 日本語
 Yorodumi
Yorodumi- EMDB-40596: Focused map of the cytoplasmic domains of rat TRPM5, conformation... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Focused map of the cytoplasmic domains of rat TRPM5, conformation high-1 | |||||||||
 Map data Map data | Focused map of the rat TRPM5 cytoplasmic domain, conformation high-1, sharpened | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / Transient Receptor Potential / TRP / Transient Receptor Potential Melastatin 5 / TRPM5 / TRANSPORT PROTEIN | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Karuppan S / Schrag LG / Jara-Oseguera A / Zubcevic L | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: Structural dynamics at cytosolic interprotomer interfaces control gating of a mammalian TRPM5 channel. Authors: Sebastian Karuppan / Lynn Goss Schrag / Caroline M Pastrano / Andrés Jara-Oseguera / Lejla Zubcevic /  Abstract: The transient receptor potential melastatin (TRPM) tetrameric cation channels are involved in a wide range of biological functions, from temperature sensing and taste transduction to regulation of ...The transient receptor potential melastatin (TRPM) tetrameric cation channels are involved in a wide range of biological functions, from temperature sensing and taste transduction to regulation of cardiac function, inflammatory pain, and insulin secretion. The structurally conserved TRPM cytoplasmic domains make up >70 % of the total protein. To investigate the mechanism by which the TRPM cytoplasmic domains contribute to gating, we employed electrophysiology and cryo-EM to study TRPM5-a channel that primarily relies on activation via intracellular Ca. Here, we show that activation of mammalian TRPM5 channels is strongly altered by Ca-dependent desensitization. Structures of rat TRPM5 identify a series of conformational transitions triggered by Ca binding, whereby formation and dissolution of cytoplasmic interprotomer interfaces appear to control activation and desensitization of the channel. This study shows the importance of the cytoplasmic assembly in TRPM5 channel function and sets the stage for future investigations of other members of the TRPM family. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40596.map.gz emd_40596.map.gz | 167.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40596-v30.xml emd-40596-v30.xml emd-40596.xml emd-40596.xml | 16.5 KB 16.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_40596.png emd_40596.png | 54.2 KB | ||
| Filedesc metadata |  emd-40596.cif.gz emd-40596.cif.gz | 4.5 KB | ||
| Others |  emd_40596_additional_1.map.gz emd_40596_additional_1.map.gz emd_40596_half_map_1.map.gz emd_40596_half_map_1.map.gz emd_40596_half_map_2.map.gz emd_40596_half_map_2.map.gz | 87.8 MB 165.1 MB 165.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40596 http://ftp.pdbj.org/pub/emdb/structures/EMD-40596 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40596 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40596 | HTTPS FTP |
-Validation report
| Summary document |  emd_40596_validation.pdf.gz emd_40596_validation.pdf.gz | 774.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_40596_full_validation.pdf.gz emd_40596_full_validation.pdf.gz | 774 KB | Display | |
| Data in XML |  emd_40596_validation.xml.gz emd_40596_validation.xml.gz | 14.5 KB | Display | |
| Data in CIF |  emd_40596_validation.cif.gz emd_40596_validation.cif.gz | 17.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40596 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40596 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40596 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40596 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40596.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40596.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused map of the rat TRPM5 cytoplasmic domain, conformation high-1, sharpened | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.12 Å | ||||||||||||||||||||||||||||||||||||
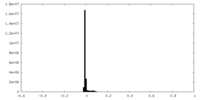
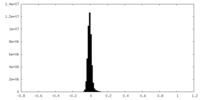
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Focused map of the rat TRPM5 cytoplasmic domain,...
| File | emd_40596_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused map of the rat TRPM5 cytoplasmic domain, conformation high-1, not sharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
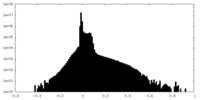
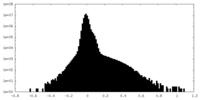
| Density Histograms |
-Half map: Focused map of the rat TRPM5 cytoplasmic domain,...
| File | emd_40596_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused map of the rat TRPM5 cytoplasmic domain, conformation high-1, half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
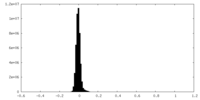
| Density Histograms |
-Half map: Focused map of the rat TRPM5 cytoplasmic domain,...
| File | emd_40596_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused map of the rat TRPM5 cytoplasmic domain, conformation high-1, half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
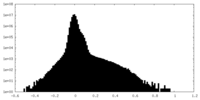
| Density Histograms |
- Sample components
Sample components
-Entire : Transient Receptor Potential Melastatin 5 (TRPM5)
| Entire | Name: Transient Receptor Potential Melastatin 5 (TRPM5) |
|---|---|
| Components |
|
-Supramolecule #1: Transient Receptor Potential Melastatin 5 (TRPM5)
| Supramolecule | Name: Transient Receptor Potential Melastatin 5 (TRPM5) / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 520 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: UltrAuFoil R1.2/1.3 / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 296.15 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.25 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: Other / Chain - Initial model type: other / Details: model generated in this study |
|---|---|
| Refinement | Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)