+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | CCT G beta 5 complex closed state 12 | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | CCT / Gb5 / complex / open / CHAPERONE | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報heterotrimeric G-protein complex assembly / GTPase activator complex / dark adaptation / light adaption / G-protein gamma-subunit binding / negative regulation of voltage-gated calcium channel activity / zona pellucida receptor complex / positive regulation of establishment of protein localization to telomere / positive regulation of protein localization to Cajal body / scaRNA localization to Cajal body ...heterotrimeric G-protein complex assembly / GTPase activator complex / dark adaptation / light adaption / G-protein gamma-subunit binding / negative regulation of voltage-gated calcium channel activity / zona pellucida receptor complex / positive regulation of establishment of protein localization to telomere / positive regulation of protein localization to Cajal body / scaRNA localization to Cajal body / positive regulation of telomerase RNA localization to Cajal body / cell tip / tubulin complex assembly / chaperonin-containing T-complex / : / BBSome-mediated cargo-targeting to cilium / Formation of tubulin folding intermediates by CCT/TriC / binding of sperm to zona pellucida / Folding of actin by CCT/TriC / regulation of G protein-coupled receptor signaling pathway / cell projection organization / positive regulation of smoothened signaling pathway / Prefoldin mediated transfer of substrate to CCT/TriC / RHOBTB1 GTPase cycle / G protein-coupled dopamine receptor signaling pathway / WD40-repeat domain binding / parallel fiber to Purkinje cell synapse / pericentriolar material / Association of TriC/CCT with target proteins during biosynthesis / positive regulation of GTPase activity / chaperone-mediated protein complex assembly / beta-tubulin binding / 加水分解酵素; 酸無水物に作用; リン含有酸無水物に作用 / RHOBTB2 GTPase cycle / heterochromatin / : / positive regulation of telomere maintenance via telomerase / visual perception / protein folding chaperone / acrosomal vesicle / Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation / GTPase activator activity / mRNA 3'-UTR binding / cell projection / ATP-dependent protein folding chaperone / mRNA 5'-UTR binding / response to virus / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through CDC42 / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / azurophil granule lumen / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / unfolded protein binding / melanosome / GPER1 signaling / Inactivation, recovery and regulation of the phototransduction cascade / protein folding / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / protein-folding chaperone binding / presynaptic membrane / cell body / Ca2+ pathway / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / secretory granule lumen / G alpha (i) signalling events / G alpha (s) signalling events / G alpha (q) signalling events / ficolin-1-rich granule lumen / microtubule / postsynaptic membrane / cytoskeleton / Extra-nuclear estrogen signaling / protein stabilization / cilium / cadherin binding / GTPase activity / dendrite / ubiquitin protein ligase binding / centrosome / Neutrophil degranulation / Golgi apparatus 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
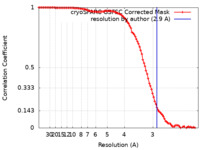
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 2.9 Å | |||||||||
 データ登録者 データ登録者 | Wang S / Sass M / Willardson BM / Shen PS | |||||||||
| 資金援助 |  米国, 1件 米国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Mol Cell / 年: 2023 ジャーナル: Mol Cell / 年: 2023タイトル: Visualizing the chaperone-mediated folding trajectory of the G protein β5 β-propeller. 著者: Shuxin Wang / Mikaila I Sass / Yujin Kwon / W Grant Ludlam / Theresa M Smith / Ethan J Carter / Nathan E Gladden / Margot Riggi / Janet H Iwasa / Barry M Willardson / Peter S Shen /  要旨: The Chaperonin Containing Tailless polypeptide 1 (CCT) complex is an essential protein folding machine with a diverse clientele of substrates, including many proteins with β-propeller domains. Here, ...The Chaperonin Containing Tailless polypeptide 1 (CCT) complex is an essential protein folding machine with a diverse clientele of substrates, including many proteins with β-propeller domains. Here, we determine the structures of human CCT in complex with its accessory co-chaperone, phosducin-like protein 1 (PhLP1), in the process of folding Gβ, a component of Regulator of G protein Signaling (RGS) complexes. Cryoelectron microscopy (cryo-EM) and image processing reveal an ensemble of distinct snapshots that represent the folding trajectory of Gβ from an unfolded molten globule to a fully folded β-propeller. These structures reveal the mechanism by which CCT directs Gβ folding through initiating specific intermolecular contacts that facilitate the sequential folding of individual β sheets until the propeller closes into its native structure. This work directly visualizes chaperone-mediated protein folding and establishes that CCT orchestrates folding by stabilizing intermediates through interactions with surface residues that permit the hydrophobic core to coalesce into its folded state. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_40492.map.gz emd_40492.map.gz | 50.2 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-40492-v30.xml emd-40492-v30.xml emd-40492.xml emd-40492.xml | 33.5 KB 33.5 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_40492_fsc.xml emd_40492_fsc.xml | 9.9 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_40492.png emd_40492.png | 391.7 KB | ||
| Filedesc metadata |  emd-40492.cif.gz emd-40492.cif.gz | 10 KB | ||
| その他 |  emd_40492_half_map_1.map.gz emd_40492_half_map_1.map.gz emd_40492_half_map_2.map.gz emd_40492_half_map_2.map.gz | 73.1 MB 73.1 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40492 http://ftp.pdbj.org/pub/emdb/structures/EMD-40492 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40492 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40492 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_40492_validation.pdf.gz emd_40492_validation.pdf.gz | 1.1 MB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_40492_full_validation.pdf.gz emd_40492_full_validation.pdf.gz | 1.1 MB | 表示 | |
| XML形式データ |  emd_40492_validation.xml.gz emd_40492_validation.xml.gz | 18.4 KB | 表示 | |
| CIF形式データ |  emd_40492_validation.cif.gz emd_40492_validation.cif.gz | 23.9 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40492 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40492 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40492 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40492 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8shqMC  8sfeC  8sffC  8sg8C  8sg9C  8sgcC  8sglC  8sgqC  8sh9C  8shaC  8shdC  8sheC  8shfC  8shgC  8shlC  8shnC  8shoC  8shpC  8shtC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_40492.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_40492.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.058 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #2
| ファイル | emd_40492_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
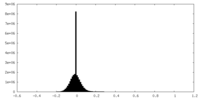
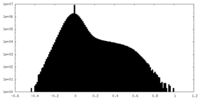
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_40492_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
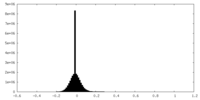
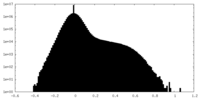
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
+全体 : CCT-Gb5-PhLP1 in closed state 12
+超分子 #1: CCT-Gb5-PhLP1 in closed state 12
+分子 #1: Phosducin-like protein
+分子 #2: Guanine nucleotide-binding protein subunit beta-5
+分子 #3: T-complex protein 1 subunit alpha
+分子 #4: T-complex protein 1 subunit beta
+分子 #5: T-complex protein 1 subunit delta
+分子 #6: T-complex protein 1 subunit epsilon
+分子 #7: T-complex protein 1 subunit gamma
+分子 #8: T-complex protein 1 subunit eta, N-terminally processed
+分子 #9: T-complex protein 1 subunit theta
+分子 #10: T-complex protein 1 subunit zeta
+分子 #11: ADENOSINE-5'-DIPHOSPHATE
+分子 #12: MAGNESIUM ION
+分子 #13: ALUMINUM FLUORIDE
+分子 #14: water
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1.5 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 緩衝液 | pH: 7.5 構成要素:
| |||||||||||||||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 277.15 K / 装置: FEI VITROBOT MARK I | |||||||||||||||||||||
| 詳細 | The sample was monodisperse |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | TFS KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 40.42 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 1.2 µm / 最小 デフォーカス(公称値): 0.8 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー















































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)