[English] 日本語
 Yorodumi
Yorodumi- EMDB-40062: Kalium channelrhodopsin 1 from Hyphochytrium catenoides (HcKCR1) ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Kalium channelrhodopsin 1 from Hyphochytrium catenoides (HcKCR1) embedded in peptidisc | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Retinal Protein / Channelrhodopsin / Cation channel / Peptidisc / Optogenetics / TRANSPORT PROTEIN | ||||||||||||
| Biological species |  Hyphochytrium catenoides (eukaryote) Hyphochytrium catenoides (eukaryote) | ||||||||||||
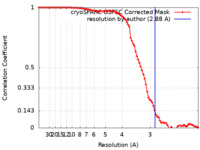
| Method | single particle reconstruction / cryo EM / Resolution: 2.88 Å | ||||||||||||
 Authors Authors | Morizumi T / Kim K / Li H / Spudich JL / Ernst OP | ||||||||||||
| Funding support |  Canada, 3 items Canada, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structures of channelrhodopsin paralogs in peptidiscs explain their contrasting K and Na selectivities. Authors: Takefumi Morizumi / Kyumhyuk Kim / Hai Li / Elena G Govorunova / Oleg A Sineshchekov / Yumei Wang / Lei Zheng / Éva Bertalan / Ana-Nicoleta Bondar / Azam Askari / Leonid S Brown / John L ...Authors: Takefumi Morizumi / Kyumhyuk Kim / Hai Li / Elena G Govorunova / Oleg A Sineshchekov / Yumei Wang / Lei Zheng / Éva Bertalan / Ana-Nicoleta Bondar / Azam Askari / Leonid S Brown / John L Spudich / Oliver P Ernst /     Abstract: Kalium channelrhodopsin 1 from Hyphochytrium catenoides (HcKCR1) is a light-gated channel used for optogenetic silencing of mammalian neurons. It selects K over Na in the absence of the canonical ...Kalium channelrhodopsin 1 from Hyphochytrium catenoides (HcKCR1) is a light-gated channel used for optogenetic silencing of mammalian neurons. It selects K over Na in the absence of the canonical tetrameric K selectivity filter found universally in voltage- and ligand-gated channels. The genome of H. catenoides also encodes a highly homologous cation channelrhodopsin (HcCCR), a Na channel with >100-fold larger Na to K permeability ratio. Here, we use cryo-electron microscopy to determine atomic structures of these two channels embedded in peptidiscs to elucidate structural foundations of their dramatically different cation selectivity. Together with structure-guided mutagenesis, we show that K versus Na selectivity is determined at two distinct sites on the putative ion conduction pathway: in a patch of critical residues in the intracellular segment (Leu69/Phe69, Ile73/Ser73 and Asp116) and within a cluster of aromatic residues in the extracellular segment (primarily, Trp102 and Tyr222). The two filters are on the opposite sides of the photoactive site involved in channel gating. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40062.map.gz emd_40062.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40062-v30.xml emd-40062-v30.xml emd-40062.xml emd-40062.xml | 20.8 KB 20.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40062_fsc.xml emd_40062_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_40062.png emd_40062.png | 43.7 KB | ||
| Filedesc metadata |  emd-40062.cif.gz emd-40062.cif.gz | 6.7 KB | ||
| Others |  emd_40062_half_map_1.map.gz emd_40062_half_map_1.map.gz emd_40062_half_map_2.map.gz emd_40062_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40062 http://ftp.pdbj.org/pub/emdb/structures/EMD-40062 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40062 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40062 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40062.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40062.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||
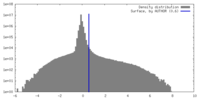
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_40062_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
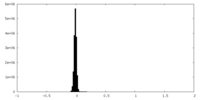
| Density Histograms |
-Half map: #1
| File | emd_40062_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
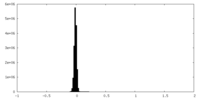
| Density Histograms |
- Sample components
Sample components
-Entire : Kalium channelrhodopsin 1 trimer reconstituted into nanodiscs
| Entire | Name: Kalium channelrhodopsin 1 trimer reconstituted into nanodiscs |
|---|---|
| Components |
|
-Supramolecule #1: Kalium channelrhodopsin 1 trimer reconstituted into nanodiscs
| Supramolecule | Name: Kalium channelrhodopsin 1 trimer reconstituted into nanodiscs type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Hyphochytrium catenoides (eukaryote) Hyphochytrium catenoides (eukaryote) |
| Molecular weight | Theoretical: 30.49385 KDa |
-Macromolecule #1: Kalium Channelrhodopsin 1
| Macromolecule | Name: Kalium Channelrhodopsin 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Hyphochytrium catenoides (eukaryote) Hyphochytrium catenoides (eukaryote) |
| Molecular weight | Theoretical: 30.515744 KDa |
| Recombinant expression | Organism:  Komagataella pastoris (fungus) Komagataella pastoris (fungus) |
| Sequence | String: MPFYDSRPPE GWPKGSINDM DYPLLGSICA VCCVFVAGSG IWMLYRLDLG MGYSCKPYKS GRAPEVNSLS GIICLLCGTM YAAKSFDFF DGGGTPFSLN WYWYLDYVFT CPLLILDFAF TLDLPHKIRY FFAVFLTLWC GVAAFVTPSA YRFAYYALGC C WFTPFALS ...String: MPFYDSRPPE GWPKGSINDM DYPLLGSICA VCCVFVAGSG IWMLYRLDLG MGYSCKPYKS GRAPEVNSLS GIICLLCGTM YAAKSFDFF DGGGTPFSLN WYWYLDYVFT CPLLILDFAF TLDLPHKIRY FFAVFLTLWC GVAAFVTPSA YRFAYYALGC C WFTPFALS LMRHVKERYL VYPPKCQRWL FWACVIFFGF WPMFPILFIF SWLGTGHISQ QAFYIIHAFL DLTCKSIFGI LM TVFRLEL EEHTEVQGLP LNEPETLS |
-Macromolecule #2: RETINAL
| Macromolecule | Name: RETINAL / type: ligand / ID: 2 / Number of copies: 1 / Formula: RET |
|---|---|
| Molecular weight | Theoretical: 284.436 Da |
| Chemical component information |  ChemComp-RET: |
-Macromolecule #3: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 3 / Number of copies: 1 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #4: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 4 / Number of copies: 3 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #5: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
| Macromolecule | Name: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine / type: ligand / ID: 5 / Number of copies: 5 / Formula: PEE |
|---|---|
| Molecular weight | Theoretical: 744.034 Da |
| Chemical component information |  ChemComp-PEE: |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 15 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.35 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: Homemade / Material: COPPER/RHODIUM / Mesh: 400 / Support film - Material: GOLD / Support film - topology: HOLEY / Support film - Film thickness: 30 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.038 kPa | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||
| Details | Sample was kept in the dark prior to freezing. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: OTHER / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 2 / Number real images: 12104 / Average electron dose: 40.0 e/Å2 Details: Images collected in movie mode, 30 fractions per movie, Falcon 4i detector |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 158.5 / Target criteria: Cross correlation coefficent |
| Output model |  PDB-8gi8: |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)