+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
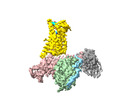
| タイトル | Cryo-EM structure of the pasireotide-bound SSTR5-Gi complex | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | pasireotide / SSTR5 / Cryo-EM / MEMBRANE PROTEIN/IMMUNE SYSTEM / MEMBRANE PROTEIN-IMMUNE SYSTEM complex | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報somatostatin receptor activity / neuropeptide binding / G-protein activation / Activation of the phototransduction cascade / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding ...somatostatin receptor activity / neuropeptide binding / G-protein activation / Activation of the phototransduction cascade / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Ca2+ pathway / positive regulation of mini excitatory postsynaptic potential / G alpha (z) signalling events / beta2-adrenergic receptor activity / negative regulation of smooth muscle contraction / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / AMPA selective glutamate receptor signaling pathway / positive regulation of autophagosome maturation / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / Adrenaline,noradrenaline inhibits insulin secretion / heat generation / ADP signalling through P2Y purinoceptor 12 / norepinephrine binding / G alpha (q) signalling events / Adrenoceptors / G alpha (i) signalling events / positive regulation of lipophagy / Thrombin signalling through proteinase activated receptors (PARs) / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / negative regulation of multicellular organism growth / negative regulation of G protein-coupled receptor signaling pathway / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / cellular response to glucocorticoid stimulus / G alpha (z) signalling events / Extra-nuclear estrogen signaling / photoreceptor outer segment membrane / G alpha (s) signalling events / G alpha (q) signalling events / endosome to lysosome transport / adrenergic receptor signaling pathway / spectrin binding / response to psychosocial stress / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / diet induced thermogenesis / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of cytokinesis / alkylglycerophosphoethanolamine phosphodiesterase activity / positive regulation of cAMP/PKA signal transduction / adenylate cyclase binding / smooth muscle contraction / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / photoreceptor outer segment / neuropeptide signaling pathway / bone resorption / potassium channel regulator activity / positive regulation of bone mineralization / neuronal dense core vesicle / brown fat cell differentiation / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / intercellular bridge / regulation of sodium ion transport / Adenylate cyclase inhibitory pathway / T cell migration / adenylate cyclase-activating adrenergic receptor signaling pathway / D2 dopamine receptor binding / response to prostaglandin E / adenylate cyclase regulator activity / G protein-coupled serotonin receptor binding / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cardiac muscle cell apoptotic process / photoreceptor inner segment / regulation of insulin secretion / cellular response to forskolin / receptor-mediated endocytosis / response to cold / regulation of mitotic spindle organization / Peptide ligand-binding receptors 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /    Oplophorus gracilirostris (甲殻類) / synthetic construct (人工物) Oplophorus gracilirostris (甲殻類) / synthetic construct (人工物) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.09 Å | |||||||||
 データ登録者 データ登録者 | Li YG / Meng XY / Yang XR / Ling SL / Shi P / Tian CL / Yang F | |||||||||
| 資金援助 | 1件
| |||||||||
 引用 引用 |  ジャーナル: Acta Pharmacol Sin / 年: 2024 ジャーナル: Acta Pharmacol Sin / 年: 2024タイトル: Structural insights into somatostatin receptor 5 bound with cyclic peptides. 著者: Ying-Ge Li / Xian-Yu Meng / Xiru Yang / Sheng-Long Ling / Pan Shi / Chang-Lin Tian / Fan Yang /  要旨: Somatostatin receptor 5 (SSTR5) is highly expressed in ACTH-secreting pituitary adenomas and is an important drug target for the treatment of Cushing's disease. Two cyclic SST analog peptides ...Somatostatin receptor 5 (SSTR5) is highly expressed in ACTH-secreting pituitary adenomas and is an important drug target for the treatment of Cushing's disease. Two cyclic SST analog peptides (pasireotide and octreotide) both can activate SSTR5 and SSTR2. Pasireotide is preferential binding to SSTR5 than octreotide, while octreotide is biased to SSTR2 than SSTR5. The lack of selectivity of both pasireotide and octreotide causes side effects, such as hyperglycemia, gastrointestinal disturbance, and abnormal glucose homeostasis. However, little is known about the binding and selectivity mechanisms of pasireotide and octreotide with SSTR5, limiting the development of subtype-selective SST analog drugs specifically targeting SSTR5. Here, we report two cryo-electron microscopy (cryo-EM) structures of SSTR5-Gi complexes activated by pasireotide and octreoitde at resolutions of 3.09 Å and 3.24 Å, respectively. In combination with structural analysis and functional experiments, our results reveal the molecular mechanisms of ligand recognition and receptor activation. We also demonstrate that pasireotide preferentially binds to SSTR5 through the interactions between Tyr(Bzl)/Trp of pasireotide and SSTR5. Moreover, we find that the Q, N, F and ECL2 of SSTR2 play a crucial role in octreotide biased binding of SSTR2. Our results will provide structural insights and offer new opportunities for the drug discovery of better selective pharmaceuticals targeting specific SSTR subtypes. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_39931.map.gz emd_39931.map.gz | 28.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-39931-v30.xml emd-39931-v30.xml emd-39931.xml emd-39931.xml | 19.5 KB 19.5 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_39931.png emd_39931.png | 52.1 KB | ||
| Filedesc metadata |  emd-39931.cif.gz emd-39931.cif.gz | 6.9 KB | ||
| その他 |  emd_39931_half_map_1.map.gz emd_39931_half_map_1.map.gz emd_39931_half_map_2.map.gz emd_39931_half_map_2.map.gz | 28.2 MB 28.2 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39931 http://ftp.pdbj.org/pub/emdb/structures/EMD-39931 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39931 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39931 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_39931_validation.pdf.gz emd_39931_validation.pdf.gz | 752.9 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_39931_full_validation.pdf.gz emd_39931_full_validation.pdf.gz | 752.5 KB | 表示 | |
| XML形式データ |  emd_39931_validation.xml.gz emd_39931_validation.xml.gz | 10.7 KB | 表示 | |
| CIF形式データ |  emd_39931_validation.cif.gz emd_39931_validation.cif.gz | 12.4 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39931 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39931 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39931 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39931 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8zcjMC  8zbeC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_39931.map.gz / 形式: CCP4 / 大きさ: 30.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_39931.map.gz / 形式: CCP4 / 大きさ: 30.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
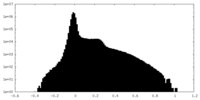
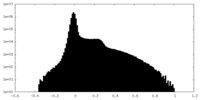
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #2
| ファイル | emd_39931_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
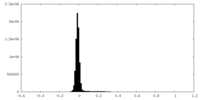
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_39931_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
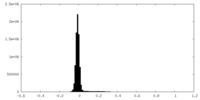
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : pasireotide-SSTR5-Gi complex
| 全体 | 名称: pasireotide-SSTR5-Gi complex |
|---|---|
| 要素 |
|
-超分子 #1: pasireotide-SSTR5-Gi complex
| 超分子 | 名称: pasireotide-SSTR5-Gi complex / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#5 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Guanine nucleotide-binding protein G(i) subunit alpha-1
| 分子 | 名称: Guanine nucleotide-binding protein G(i) subunit alpha-1 タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 40.445059 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKSTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...文字列: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKSTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVGAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHESM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCS TDTKNVQFVF DAVTDVIIKN NLKDCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-分子 #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 41.055867 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI ...文字列: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI VTSSGDTTCA LWDIETGQQT TTFTGHTGDV MSLSLAPDTR LFVSGACDAS AKLWDVREGM CRQTFTGHES DI NAICFFP NGNAFATGSD DATCRLFDLR ADQELMTYSH DNIICGITSV SFSKSGRLLL AGYDDFNCNV WDALKADRAG VLA GHDNRV SCLGVTDDGM AVATGSWDSF LKIWNGSSGG GGSGGGGSSG VSGWRLFKKI S UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-分子 #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 7.861143 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-分子 #4: ScFv16
| 分子 | 名称: ScFv16 / タイプ: protein_or_peptide / ID: 4 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 32.724473 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSSG G GGSGGGGS ...文字列: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSSG G GGSGGGGS GGGGSSDIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLELVD ENLYFQGASH HHHHHHH |
-分子 #5: Beta-2 adrenergic receptor,Somatostatin receptor type 5,lgbit (fu...
| 分子 | 名称: Beta-2 adrenergic receptor,Somatostatin receptor type 5,lgbit (fusion protein) タイプ: protein_or_peptide / ID: 5 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Oplophorus gracilirostris (甲殻類) Oplophorus gracilirostris (甲殻類) |
| 分子量 | 理論値: 62.01593 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MKTIIALSYI FCLVFADYKD DDDKMGQPGN GSAFLLAPNG SHAPDHDVME PLFPASTPSW NASSPGAASG GGDNRTLVGP APSAGARAV LVPVLYLLVC AAGLGGNTLV IYVVLRFAKM KTVTNIYILN LAVADVLYML GLPFLATQNA ASFWPFGPVL C RLVMTLDG ...文字列: MKTIIALSYI FCLVFADYKD DDDKMGQPGN GSAFLLAPNG SHAPDHDVME PLFPASTPSW NASSPGAASG GGDNRTLVGP APSAGARAV LVPVLYLLVC AAGLGGNTLV IYVVLRFAKM KTVTNIYILN LAVADVLYML GLPFLATQNA ASFWPFGPVL C RLVMTLDG VNQFTSVFCL TVMSVDRYLA VVHPLSSARW RRPRVAKLAS AAAWVLSLCM SLPLLVFADV QEGGTCNASW PE PVGLWGA VFIIYTAVLG FFAPLLVICL CYLLIVVKVR AAGVRVGCVR RRSERKVTRM VLVVVLVFAG CWLPFFTVNI VNL AVALPQ EPASAGLYFF VVILSYANSC ANPVLYGFLS DNFRQSFQKV LCLRKGSGAK DADATEPRPD RIRQQQEATP PAHR AAANG LMQTSKLVFT LEDFVGDWEQ TAAYNLDQVL EQGGVSSLLQ NLAVSVTPIQ RIVRSGENAL KIDIHVIIPY EGLSA DQMA QIEEVFKVVY PVDDHHFKVI LPYGTLVIDG VTPNMLNYFG RPYEGIAVFD GKKITVTGTL WNGNKIIDER LITPDG SML FRVTINS UniProtKB: Beta-2 adrenergic receptor, Somatostatin receptor type 5 |
-分子 #6: 004-DTR-LYS-TYR-PHA-HYP
| 分子 | 名称: 004-DTR-LYS-TYR-PHA-HYP / タイプ: protein_or_peptide / ID: 6 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種: synthetic construct (人工物) |
| 分子量 | 理論値: 874.016 Da |
| 配列 | 文字列: (004)(DTR)KY(PHA)(HYP) |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 55.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: SPOT SCAN / 撮影モード: DIFFRACTION / 最大 デフォーカス(公称値): 2.0 µm 最小 デフォーカス(公称値): 1.4000000000000001 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: PDB ENTRY PDBモデル - PDB ID: |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.09 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 1227503 |
| 初期 角度割当 | タイプ: ANGULAR RECONSTITUTION |
| 最終 角度割当 | タイプ: ANGULAR RECONSTITUTION |
 ムービー
ムービー コントローラー
コントローラー




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)