+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | pP1192R-DNA-m-AMSA complex DNA binding/cleavage domain | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ASFV / PROTEIN BINDING / ISOMERASE-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsister chromatid segregation / DNA topoisomerase type II (double strand cut, ATP-hydrolyzing) activity / DNA topoisomerase (ATP-hydrolysing) / DNA topological change / host cell cytoplasm / DNA binding / ATP binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  African swine fever virus pig/Kenya/KEN-50/1950 African swine fever virus pig/Kenya/KEN-50/1950 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.76 Å | |||||||||
 Authors Authors | Sun JQ / Liu RL | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2024 Journal: Nucleic Acids Res / Year: 2024Title: Structural basis for difunctional mechanism of m-AMSA against African swine fever virus pP1192R. Authors: Ruili Liu / Junqing Sun / Lian-Feng Li / Yingxian Cheng / Meilin Li / Lifeng Fu / Su Li / Guorui Peng / Yanjin Wang / Sheng Liu / Xiao Qu / Jiaqi Ran / Xiaomei Li / Erqi Pang / Hua-Ji Qiu / ...Authors: Ruili Liu / Junqing Sun / Lian-Feng Li / Yingxian Cheng / Meilin Li / Lifeng Fu / Su Li / Guorui Peng / Yanjin Wang / Sheng Liu / Xiao Qu / Jiaqi Ran / Xiaomei Li / Erqi Pang / Hua-Ji Qiu / Yanli Wang / Jianxun Qi / Han Wang / George Fu Gao /  Abstract: The African swine fever virus (ASFV) type II topoisomerase (Topo II), pP1192R, is the only known Topo II expressed by mammalian viruses and is essential for ASFV replication in the host cytoplasm. ...The African swine fever virus (ASFV) type II topoisomerase (Topo II), pP1192R, is the only known Topo II expressed by mammalian viruses and is essential for ASFV replication in the host cytoplasm. Herein, we report the structures of pP1192R in various enzymatic stages using both X-ray crystallography and single-particle cryo-electron microscopy. Our data structurally define the pP1192R-modulated DNA topology changes. By presenting the A2+-like metal ion at the pre-cleavage site, the pP1192R-DNA-m-AMSA complex structure provides support for the classical two-metal mechanism in Topo II-mediated DNA cleavage and a better explanation for nucleophile formation. The unique inhibitor selectivity of pP1192R and the difunctional mechanism of pP1192R inhibition by m-AMSA highlight the specificity of viral Topo II in the poison binding site. Altogether, this study provides the information applicable to the development of a pP1192R-targeting anti-ASFV strategy. | |||||||||
| History |
|
- Structure visualization
Structure visualization
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39245.map.gz emd_39245.map.gz | 51.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39245-v30.xml emd-39245-v30.xml emd-39245.xml emd-39245.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_39245.png emd_39245.png | 47.6 KB | ||
| Filedesc metadata |  emd-39245.cif.gz emd-39245.cif.gz | 7.2 KB | ||
| Others |  emd_39245_half_map_1.map.gz emd_39245_half_map_1.map.gz emd_39245_half_map_2.map.gz emd_39245_half_map_2.map.gz | 95.7 MB 95.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39245 http://ftp.pdbj.org/pub/emdb/structures/EMD-39245 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39245 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39245 | HTTPS FTP |
-Related structure data
| Related structure data |  8ygeMC  8yggC  8yghC  8yikC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39245.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39245.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : pP1192R-DNA-m-AMSA complex DNA binding/cleavage domain
| Entire | Name: pP1192R-DNA-m-AMSA complex DNA binding/cleavage domain |
|---|---|
| Components |
|
-Supramolecule #1: pP1192R-DNA-m-AMSA complex DNA binding/cleavage domain
| Supramolecule | Name: pP1192R-DNA-m-AMSA complex DNA binding/cleavage domain type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  African swine fever virus pig/Kenya/KEN-50/1950 African swine fever virus pig/Kenya/KEN-50/1950 |
-Macromolecule #1: DNA topoisomerase 2
| Macromolecule | Name: DNA topoisomerase 2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: DNA topoisomerase (ATP-hydrolysing) |
|---|---|
| Source (natural) | Organism:  African swine fever virus pig/Kenya/KEN-50/1950 African swine fever virus pig/Kenya/KEN-50/1950 |
| Molecular weight | Theoretical: 135.859891 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: METFEISDFK EHAKKKSMWA GALNKVTISG LMGVFTEDED LMALPIHRDH CPALLKIFDE LIVNATDHER ACHNKTKKVT YIKISFDKG VFSCENDGPG IPIVKHEQAS LIAKRDVYVP EVASCYFLAG TNINKAKDCI KGGTNGVGLK LAMVHSQWAI L TTADGSQK ...String: METFEISDFK EHAKKKSMWA GALNKVTISG LMGVFTEDED LMALPIHRDH CPALLKIFDE LIVNATDHER ACHNKTKKVT YIKISFDKG VFSCENDGPG IPIVKHEQAS LIAKRDVYVP EVASCYFLAG TNINKAKDCI KGGTNGVGLK LAMVHSQWAI L TTADGSQK YVQHINQRLD NIEPPTITPS REMFTRIELM PVYQELGYAQ PLSETEQADL SAWIYLRACQ CAAYVGKGTT IY YNDKPCS TSSVMALAKM YTLVTAPNST IYTATIKADA KPYSLHPLQV AAVVSPKFKK FEHVSIINGV NCVKGEHVTF LKK AINEMV VKKFQQTIKD KNRKTTLRDS CSNIFVVIVG SIPGIEWTGQ RKDELSIAEN VFKTHYSIPS SFLTSMTRSI VDIL LQSIS KKDNHKQIDV DKYTRARNAG GKKAQDCMLL AAEGDSALSL LRAGLTLGKS NPSGPSFDFC GMISLGGVIM NACKK VTNI TTDSGETIMV RNEQLTNNKV LQGIVQVLGL DFNCHYKTQE ERAKLRYGCI VACVDQDLDG CGKILGLLLA YFHLFW PQL IVHGFVKRLL TPLIRVYEKG NTVPVEFYYE QEFDAWAKKQ TSLANHTVKY YKGLAAHDTH EVKSMFKHFD KMVYTFT LD DSAKELFHIY FGGESELRKR ELCTGVVPLT ETQTQSIHSV RRIPCSLHLQ VDTKAYKLDA IERQIPNFLD GMTRARRK I LAGGLKCFAS NNRERKVFQF GGYVADHMFY HHGDMSLNTS IIKAAQYYPG SSHLYPVFIG IGSFGSRHLG GKDAGSPRY ISVQLASEFI KTMFPTEDSW LLPYVFEDGQ RAEPEYYVPV LPLAIMEYGA NPSEGWKYTT WARQLEDILA LVRAYVDKNN PKHELLHYA IDHKITVLPL RPSNYNFKGH LKRFGQYYYS YGTYVVSEQR NMITITELPL RVPTVAYIES IKKSSNRMAF I EEIVDYSS SETIEILVKL KPNSLSRIME EFKETEEQNS IENFLRLRNC LHSHLNFVKP KGGIIEFNSY YEILYAWLPY RR DLYQKRL MRERAVLKLR LIMETAIVRY INESADLNLS HYEDEKEAGR ILSEHGFPPL NQSLITSPEF ATIEELNQKA LQG CYTYIL SLQARELLIA AKTRRVEKIK KMQARLDKVE QLLQESPFPG ASVWLEEIDA VEKAIIKGRN TQWKFHHH UniProtKB: DNA topoisomerase 2 |
-Macromolecule #2: DNA (20-mer)
| Macromolecule | Name: DNA (20-mer) / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  African swine fever virus pig/Kenya/KEN-50/1950 African swine fever virus pig/Kenya/KEN-50/1950 |
| Molecular weight | Theoretical: 6.189014 KDa |
| Sequence | String: (DG)(DA)(DT)(DT)(DG)(DG)(DA)(DT)(DA)(DG) (DC)(DT)(DA)(DT)(DT)(DC)(DA)(DC)(DG)(DG) |
-Macromolecule #3: DNA (8-mer)
| Macromolecule | Name: DNA (8-mer) / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  African swine fever virus pig/Kenya/KEN-50/1950 African swine fever virus pig/Kenya/KEN-50/1950 |
| Molecular weight | Theoretical: 2.426617 KDa |
| Sequence | String: (DC)(DC)(DG)(DT)(DG)(DA)(DA)(DT) |
-Macromolecule #4: DNA (12-mer)
| Macromolecule | Name: DNA (12-mer) / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  African swine fever virus pig/Kenya/KEN-50/1950 African swine fever virus pig/Kenya/KEN-50/1950 |
| Molecular weight | Theoretical: 3.606381 KDa |
| Sequence | String: (DA)(DG)(DC)(DT)(DA)(DT)(DC)(DC)(DA)(DA) (DT)(DC) |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 3 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: N-[4-(acridin-9-ylamino)-3-methoxyphenyl]methanesulfonamide
| Macromolecule | Name: N-[4-(acridin-9-ylamino)-3-methoxyphenyl]methanesulfonamide type: ligand / ID: 6 / Number of copies: 2 / Formula: ASW |
|---|---|
| Molecular weight | Theoretical: 393.459 Da |
| Chemical component information | 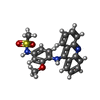 ChemComp-ASW: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 20mM NaCl,150mM Tris-HCl |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.001 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















