[English] 日本語
 Yorodumi
Yorodumi- EMDB-39238: The early intermediate structure of baculovirus fusion protein GP64 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The early intermediate structure of baculovirus fusion protein GP64 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated perturbation of host process / membrane fusion involved in viral entry into host cell / viral budding from plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / host cell plasma membrane / virion membrane / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  Autographa californica nucleopolyhedrovirus Autographa californica nucleopolyhedrovirus | |||||||||
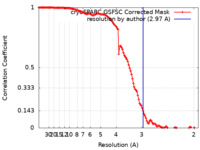
| Method | single particle reconstruction / cryo EM / Resolution: 2.97 Å | |||||||||
 Authors Authors | Du D / Guo J / Li S | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural transition of GP64 triggered by a pH-sensitive multi-histidine switch. Authors: Jinliang Guo / Shangrong Li / Lisha Bai / Huimin Zhao / Wenyu Shang / Zhaojun Zhong / Tuerxunjiang Maimaiti / Xueyan Gao / Ning Ji / Yanjie Chao / Zhaofei Li / Dijun Du /  Abstract: The fusion of viruses with cellular membranes is a critical step in the life cycle of enveloped viruses. This process is facilitated by viral fusion proteins, many of which are conformationally pH- ...The fusion of viruses with cellular membranes is a critical step in the life cycle of enveloped viruses. This process is facilitated by viral fusion proteins, many of which are conformationally pH-sensitive. The specifics of how changes in pH initiate this fusion have remained largely elusive. This study presents the cryo-electron microscopy (cryo-EM) structures of a prototype class III fusion protein, GP64, in its prefusion and early intermediate states, revealing the structural intermediates accompanying the membrane fusion process. The structures identify the involvement of a pH-sensitive switch, comprising H23, H245, and H304, in sensing the low pH that triggers the initial step of membrane fusion. The pH sensing role of this switch is corroborated by assays of cell-cell syncytium formation and dual dye-labeling. The findings demonstrate that coordination between multiple histidine residues acts as a pH sensor and activator. The involvement of a multi-histidine switch in viral fusion is applicable to fusogens of human-infecting thogotoviruses and other viruses, which could lead to strategies for developing anti-viral therapies and vaccines. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39238.map.gz emd_39238.map.gz | 2.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39238-v30.xml emd-39238-v30.xml emd-39238.xml emd-39238.xml | 17.7 KB 17.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_39238_fsc.xml emd_39238_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_39238.png emd_39238.png | 54.8 KB | ||
| Masks |  emd_39238_msk_1.map emd_39238_msk_1.map | 178 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-39238.cif.gz emd-39238.cif.gz | 6.4 KB | ||
| Others |  emd_39238_half_map_1.map.gz emd_39238_half_map_1.map.gz emd_39238_half_map_2.map.gz emd_39238_half_map_2.map.gz | 165 MB 165 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39238 http://ftp.pdbj.org/pub/emdb/structures/EMD-39238 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39238 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39238 | HTTPS FTP |
-Validation report
| Summary document |  emd_39238_validation.pdf.gz emd_39238_validation.pdf.gz | 704.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39238_full_validation.pdf.gz emd_39238_full_validation.pdf.gz | 703.9 KB | Display | |
| Data in XML |  emd_39238_validation.xml.gz emd_39238_validation.xml.gz | 20.7 KB | Display | |
| Data in CIF |  emd_39238_validation.cif.gz emd_39238_validation.cif.gz | 26.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39238 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39238 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39238 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39238 | HTTPS FTP |
-Related structure data
| Related structure data |  8yg8MC  8yg6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_39238.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39238.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.96 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_39238_msk_1.map emd_39238_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : GP64
| Entire | Name: GP64 |
|---|---|
| Components |
|
-Supramolecule #1: GP64
| Supramolecule | Name: GP64 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Autographa californica nucleopolyhedrovirus Autographa californica nucleopolyhedrovirus |
-Macromolecule #1: Major envelope glycoprotein
| Macromolecule | Name: Major envelope glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Autographa californica nucleopolyhedrovirus Autographa californica nucleopolyhedrovirus |
| Molecular weight | Theoretical: 53.338871 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HCNAQMKTGP YKIKNLDITP PKETLQKDVE ITIVETDYNE NVIIGYKGYY QAYAYNGGSL DPNTRVEETM KTLNVGKEDL LMWSIRQQC EVGEELIDRW GSDSDDCFRD NEGRGQWVKG KELVKRQNNN HFAHHTCNKS WRCGISTSKM YSRLECQDDT D ECQVYILD ...String: HCNAQMKTGP YKIKNLDITP PKETLQKDVE ITIVETDYNE NVIIGYKGYY QAYAYNGGSL DPNTRVEETM KTLNVGKEDL LMWSIRQQC EVGEELIDRW GSDSDDCFRD NEGRGQWVKG KELVKRQNNN HFAHHTCNKS WRCGISTSKM YSRLECQDDT D ECQVYILD AEGNPINVTV DTVLHRDGVS MILKQKSTFT TRQIKAACLL IKDDKNNPES VTREHCLIDN DIYDLSKNTW NC KFNRCIK RKVEHRVKKR PPTWRHNVRA KYTEGDTATK GDLMHIQEEL MYENDLLKMN IELMHAHINK LNNMLHDLIV SVA KVDERL IGNLMNNSVS STFLSDDTFL LMPCTNPPAH TSNCYNNSIY KEGRWVANTD SSQCIDFSNY KELAIDDDVE FWIP TIGNT TYHDSWKDAS GWSFIAQQKS NLITTMENTK FGGVGTSLSD ITSMAEGELA AKLTSFMFGH UniProtKB: Major envelope glycoprotein |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 7 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 60.02 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)