[English] 日本語
 Yorodumi
Yorodumi- EMDB-38936: Cryo-EM structure of E.coli spermidine transporter PotD-PotABC in... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of E.coli spermidine transporter PotD-PotABC in translocation intermidiate state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC transporter / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationABC-type polyamine transporter / ABC-type putrescine transporter activity / spermidine transmembrane transport / polyamine binding / polyamine transport / ABC-type polyamine transporter activity / putrescine transport / ATP-binding cassette (ABC) transporter complex / outer membrane-bounded periplasmic space / nucleotide binding ...ABC-type polyamine transporter / ABC-type putrescine transporter activity / spermidine transmembrane transport / polyamine binding / polyamine transport / ABC-type polyamine transporter activity / putrescine transport / ATP-binding cassette (ABC) transporter complex / outer membrane-bounded periplasmic space / nucleotide binding / ATP hydrolysis activity / ATP binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Qiao Z / Gao YG | |||||||||
| Funding support |  Singapore, 1 items Singapore, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2024 Journal: Sci Adv / Year: 2024Title: Structural insights into polyamine spermidine uptake by the ABC transporter PotD-PotABC. Authors: Zhu Qiao / Phong Hoa Do / Joshua Yi Yeo / Rya Ero / Zhuowen Li / Liying Zhan / Sandip Basak / Yong-Gui Gao /   Abstract: Polyamines, characterized by their polycationic nature, are ubiquitously present in all organisms and play numerous cellular functions. Among polyamines, spermidine stands out as the predominant type ...Polyamines, characterized by their polycationic nature, are ubiquitously present in all organisms and play numerous cellular functions. Among polyamines, spermidine stands out as the predominant type in both prokaryotic and eukaryotic cells. The PotD-PotABC protein complex in , belonging to the adenosine triphosphate-binding cassette transporter family, is a spermidine-preferential uptake system. Here, we report structural details of the polyamine uptake system PotD-PotABC in various states. Our analyses reveal distinct "inward-facing" and "outward-facing" conformations of the PotD-PotABC transporter, as well as conformational changes in the "gating" residues (F222, Y223, D226, and K241 in PotB; Y219 and K223 in PotC) controlling spermidine uptake. Therefore, our structural analysis provides insights into how the PotD-PotABC importer recognizes the substrate-binding protein PotD and elucidates molecular insights into the spermidine uptake mechanism of bacteria. | |||||||||
| History |
|
- Structure visualization
Structure visualization
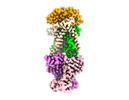
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38936.map.gz emd_38936.map.gz | 15.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38936-v30.xml emd-38936-v30.xml emd-38936.xml emd-38936.xml | 18 KB 18 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_38936.png emd_38936.png | 60.8 KB | ||
| Filedesc metadata |  emd-38936.cif.gz emd-38936.cif.gz | 7.1 KB | ||
| Others |  emd_38936_half_map_1.map.gz emd_38936_half_map_1.map.gz emd_38936_half_map_2.map.gz emd_38936_half_map_2.map.gz | 28.4 MB 28.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38936 http://ftp.pdbj.org/pub/emdb/structures/EMD-38936 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38936 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38936 | HTTPS FTP |
-Related structure data
| Related structure data |  8y5iMC  8y5fC  8y5gC  8y5hC  8zx1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_38936.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38936.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.216 Å | ||||||||||||||||||||||||||||||||||||
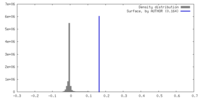
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_38936_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
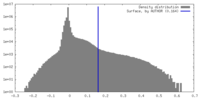
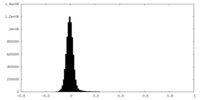
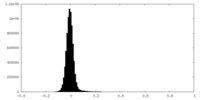
| Density Histograms |
-Half map: #1
| File | emd_38936_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
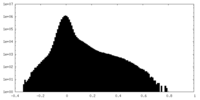
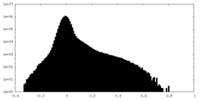
| Density Histograms |
- Sample components
Sample components
-Entire : ABC transporter
| Entire | Name: ABC transporter |
|---|---|
| Components |
|
-Supramolecule #1: ABC transporter
| Supramolecule | Name: ABC transporter / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 186.1 kDa/nm |
-Macromolecule #1: Spermidine/putrescine import ATP-binding protein PotA
| Macromolecule | Name: Spermidine/putrescine import ATP-binding protein PotA / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: ABC-type polyamine transporter |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 43.079137 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGQSKKLNKQ PSSLSPLVQL AGIRKCFDGK EVIPQLDLTI NNGEFLTLLG PSGCGKTTVL RLIAGLETVD SGRIMLDNED ITHVPAENR YVNTVFQSYA LFPHMTVFEN VAFGLRMQKT PAAEITPRVM EALRMVQLET FAQRKPHQLS GGQQQRVAIA R AVVNKPRL ...String: MGQSKKLNKQ PSSLSPLVQL AGIRKCFDGK EVIPQLDLTI NNGEFLTLLG PSGCGKTTVL RLIAGLETVD SGRIMLDNED ITHVPAENR YVNTVFQSYA LFPHMTVFEN VAFGLRMQKT PAAEITPRVM EALRMVQLET FAQRKPHQLS GGQQQRVAIA R AVVNKPRL LLLDQSLSAL DYKLRKQMQN ELKALQRKLG ITFVFVTHDQ EEALTMSDRI VVMRDGRIEQ DGTPREIYEE PK NLFVAGF IGEINMFNAT VIERLDEQRV RANVEGRECN IYVNFAVEPG QKLHVLLRPE DLRVEEINDD NHAEGLIGYV RER NYKGMT LESVVELENG KMVMVSEFFN EDDPDFDHSL DQKMAINWVE SWEVVLADEE HK UniProtKB: Spermidine/putrescine import ATP-binding protein PotA |
-Macromolecule #2: Spermidine/putrescine transport system permease protein PotB
| Macromolecule | Name: Spermidine/putrescine transport system permease protein PotB type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 32.20609 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKNTTSFQNV VIVTIVGWLV LFVFLPNLMI IGTSFLTRDD ASFVKMVFTL DNYTRLLDPL YFEVLLHSLN MALIATLACL VLGYPFAWF LAKLPHKVRP LLLFLLIVPF WTNSLIRIYG LKIFLSTKGY LNEFLLWLGV IDTPIRIMFT PSAVIIGLVY I LLPFMVMP ...String: MKNTTSFQNV VIVTIVGWLV LFVFLPNLMI IGTSFLTRDD ASFVKMVFTL DNYTRLLDPL YFEVLLHSLN MALIATLACL VLGYPFAWF LAKLPHKVRP LLLFLLIVPF WTNSLIRIYG LKIFLSTKGY LNEFLLWLGV IDTPIRIMFT PSAVIIGLVY I LLPFMVMP LYSSIEKLDK PLLEAARDLG ASKLQTFIRI IIPLTMPGII AGCLLVMLPA MGLFYVSDLM GGAKNLLIGN VI KVQFLNI RDWPFGAATS ITLTIVMGLM LLVYWRASRL LNKKVELE |
-Macromolecule #3: Spermidine/putrescine transport system permease protein PotC
| Macromolecule | Name: Spermidine/putrescine transport system permease protein PotC type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 29.13285 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIGRLLRGGF MTAIYAYLYI PIIILIVNSF NSSRFGINWQ GFTTKWYSLL MNNDSLLQAA QHSLTMAVFS ATFATLIGSL TAVALYRYR FRGKPFVSGM LFVVMMSPDI VMAISLLVLF MLLGIQLGFW SLLFSHITFC LPFVVVTVYS RLKGFDVRML E AAKDLGAS ...String: MIGRLLRGGF MTAIYAYLYI PIIILIVNSF NSSRFGINWQ GFTTKWYSLL MNNDSLLQAA QHSLTMAVFS ATFATLIGSL TAVALYRYR FRGKPFVSGM LFVVMMSPDI VMAISLLVLF MLLGIQLGFW SLLFSHITFC LPFVVVTVYS RLKGFDVRML E AAKDLGAS EFTILRKIIL PLAMPAVAAG WVLSFTLSMD DVVVSSFVTG PSYEILPLKI YSMVKVGVSP EVNALATILL VL SLVMVIA SQLIARDKTK GNTGDVK UniProtKB: Spermidine/putrescine transport system permease protein PotC |
-Macromolecule #4: Putrescine-binding periplasmic protein
| Macromolecule | Name: Putrescine-binding periplasmic protein / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 38.906938 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKKWSRHLLA AGALALGMSA AHADDNNTLY FYNWTEYVPP GLLEQFTKET GIKVIYSTYE SNETMYAKLK TYKDGAYDLV VPSTYYVDK MRKEGMIQKI DKSKLTNFSN LDPDMLNKPF DPNNDYSIPY IWGATAIGVN GDAVDPKSVT SWADLWKPEY K GSLLLTDD ...String: MKKWSRHLLA AGALALGMSA AHADDNNTLY FYNWTEYVPP GLLEQFTKET GIKVIYSTYE SNETMYAKLK TYKDGAYDLV VPSTYYVDK MRKEGMIQKI DKSKLTNFSN LDPDMLNKPF DPNNDYSIPY IWGATAIGVN GDAVDPKSVT SWADLWKPEY K GSLLLTDD AREVFQMALR KLGYSGNTTD PKEIEAAYNE LKKLMPNVAA FNSDNPANPY MEGEVNLGMI WNGSAFVARQ AG TPIDVVW PKEGGIFWMD SLAIPANAKN KEGALKLINF LLRPDVAKQV AETIGYPTPN LAARKLLSPE VANDKTLYPD AET IKNGEW QNDVGAASSI YEEYYQKLKA GR UniProtKB: Putrescine-binding periplasmic protein |
-Macromolecule #5: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 2 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #6: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 6 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 107909 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)