[English] 日本語
 Yorodumi
Yorodumi- EMDB-38301: Cryo-EM structure of the ATP-bound Mtb DppABCD with the D445A mut... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the ATP-bound Mtb DppABCD with the D445A mutation of DppA | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DppABCD / ABC importer / Mycobacterium tuberculosis / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpeptide transport / peptide transmembrane transporter activity / transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex / transmembrane transport / periplasmic space / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Mycobacterium tuberculosis H37Rv (bacteria) / Mycobacterium tuberculosis H37Rv (bacteria) /  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.89 Å | |||||||||
 Authors Authors | Hu T / Zhang B / Rao Z | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM structure of the ATP-bound Mtb DppABCD with the D445A mutation of DppA Authors: Hu T / Zhang B / Rao Z | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38301.map.gz emd_38301.map.gz | 203.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38301-v30.xml emd-38301-v30.xml emd-38301.xml emd-38301.xml | 20.3 KB 20.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_38301.png emd_38301.png | 81 KB | ||
| Filedesc metadata |  emd-38301.cif.gz emd-38301.cif.gz | 6.7 KB | ||
| Others |  emd_38301_half_map_1.map.gz emd_38301_half_map_1.map.gz emd_38301_half_map_2.map.gz emd_38301_half_map_2.map.gz | 200.7 MB 200.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38301 http://ftp.pdbj.org/pub/emdb/structures/EMD-38301 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38301 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38301 | HTTPS FTP |
-Validation report
| Summary document |  emd_38301_validation.pdf.gz emd_38301_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_38301_full_validation.pdf.gz emd_38301_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_38301_validation.xml.gz emd_38301_validation.xml.gz | 15.6 KB | Display | |
| Data in CIF |  emd_38301_validation.cif.gz emd_38301_validation.cif.gz | 18.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38301 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38301 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38301 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38301 | HTTPS FTP |
-Related structure data
| Related structure data |  8xfcMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_38301.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38301.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||
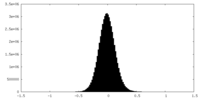
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_38301_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
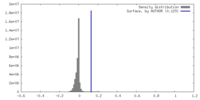
| Density Histograms |
-Half map: #1
| File | emd_38301_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
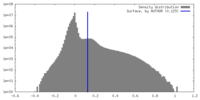
| Density Histograms |
- Sample components
Sample components
-Entire : DppABCD
| Entire | Name: DppABCD |
|---|---|
| Components |
|
-Supramolecule #1: DppABCD
| Supramolecule | Name: DppABCD / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) |
-Macromolecule #1: Probable dipeptide-transport integral membrane protein ABC transp...
| Macromolecule | Name: Probable dipeptide-transport integral membrane protein ABC transporter DppB type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) |
| Molecular weight | Theoretical: 32.563557 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Sequence | String: MGWYVARRVA VMVPVFLGAT LLIYGMVFLL PGDPVAALAG DRPLTPAVAA QLRSHYHLDD PFLVQYLRYL GGILHGDLGR AYSGLPVSA VLAHAFPVTI RLALIALAVE AVLGIGFGVI AGLRQGGIFD SAVLVTGLVI IAIPIFVLGF LAQFLFGVQL E IAPVTVGE ...String: MGWYVARRVA VMVPVFLGAT LLIYGMVFLL PGDPVAALAG DRPLTPAVAA QLRSHYHLDD PFLVQYLRYL GGILHGDLGR AYSGLPVSA VLAHAFPVTI RLALIALAVE AVLGIGFGVI AGLRQGGIFD SAVLVTGLVI IAIPIFVLGF LAQFLFGVQL E IAPVTVGE RASVGRLLLP GIVLGAMSFA YVVRLTRSAV AANAHADYVR TATAKGLSRP RVVTVHILRN SLIPVVTFLG AD LGALMGG AIVTEGIFNI HGVGGVLYQA VTRQETPTVV SIVTVLVLIY LITNLLVDLL YAALDPRIRY G UniProtKB: Probable dipeptide-transport integral membrane protein ABC transporter DppB |
-Macromolecule #2: Probable dipeptide-transport integral membrane protein ABC transp...
| Macromolecule | Name: Probable dipeptide-transport integral membrane protein ABC transporter DppC type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) |
| Molecular weight | Theoretical: 30.48573 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Sequence | String: MAEHTGFWLD AWRGLRRRPK FVIAAALILL ILVVAAFPSL FTAADPTYAD PSQSMLAPSA AHWFGTDLQG HDIYSRTVYG ARASVTVGL GATLAVFVVG GALGALAGFY GSWIDAVVSR VTDVFLGLPL LLAAIVLMQV MHHRTVWTVI AILALFGWPQ V ARIARGAV ...String: MAEHTGFWLD AWRGLRRRPK FVIAAALILL ILVVAAFPSL FTAADPTYAD PSQSMLAPSA AHWFGTDLQG HDIYSRTVYG ARASVTVGL GATLAVFVVG GALGALAGFY GSWIDAVVSR VTDVFLGLPL LLAAIVLMQV MHHRTVWTVI AILALFGWPQ V ARIARGAV LEVRASDYVL AAKALGLNRF QILLRHALPN AVGPVIAVAT VALGIFIVTE ATLSYLGVGL PTSVVSWGGD IN VAQTRLR SGSPILFYPA GALAITVLAF MMMGDALRDA LDPASRAWRA UniProtKB: Probable dipeptide-transport integral membrane protein ABC transporter DppC |
-Macromolecule #3: Probable dipeptide-transport ATP-binding protein ABC transporter DppD
| Macromolecule | Name: Probable dipeptide-transport ATP-binding protein ABC transporter DppD type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) |
| Molecular weight | Theoretical: 59.018547 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Sequence | String: MSVPAAPLLS VEGLEVTFGT DAPAVCGVDL AVRSGQTVAV VGESGSGKST TAAAILGLLP AGGRITAGRV VFDGRDITGA DAKRLRSIR GREIGYVPQD PMTNLNPVWK VGFQVTEALR ANTDGRAARR RAVELLAEAG LPDPAKQAGR YPHQLSGGMC Q RALIAIGL ...String: MSVPAAPLLS VEGLEVTFGT DAPAVCGVDL AVRSGQTVAV VGESGSGKST TAAAILGLLP AGGRITAGRV VFDGRDITGA DAKRLRSIR GREIGYVPQD PMTNLNPVWK VGFQVTEALR ANTDGRAARR RAVELLAEAG LPDPAKQAGR YPHQLSGGMC Q RALIAIGL AGRPRLLIAD QPTSALDVTV QRQVLDHLQG LTDELGTALL LITHDLALAA QRAEAVVVVR RGVVVESGAA QS ILQSPQH EYTRRLVAAA PSLTARSRRP PESRSRATTQ AGDILVVSEL TKIYRESRGA PWRRVESRAV DGVSFRLPRA STL AIVGES GSGKSTLARM VLGLLQPTSG TVVFDGTYDV GALARDQVLA FRRRVQPVFQ NPYSSLDPMY SVFRAIEEPL RVHH VGDRR QRQRAVRELV DQVALPSSIL GRRPRELSGG QRQRVAIARA LALRPEVLVC DQAVSALDVL VQAQILDLLA DLQAD LGLT YLFISHDLAV IRQIADDVLV MRAGRVVEHA STEEVFSRPR HEYTRQLLQA IPGAPSAPRK VGNL UniProtKB: Probable dipeptide-transport ATP-binding protein ABC transporter DppD |
-Macromolecule #4: Probable periplasmic dipeptide-binding lipoprotein DppA
| Macromolecule | Name: Probable periplasmic dipeptide-binding lipoprotein DppA type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) |
| Molecular weight | Theoretical: 56.013234 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Sequence | String: CGGGVLSPDV VLVNGGEPPN PLIPTGTNDS NGGRIIDRLF AGLMSYDAVG KPSLEVAQSI ESADNVNYRI TVKPGWKFTD GSPVTAHSF VDAWNYGALS TNAQLQQHFF SPIEGFDDVA GAPGDKSRTT MSGLRVVNDL EFTVRLKAPT IDFTLRLGHS S FYPLPDSA ...String: CGGGVLSPDV VLVNGGEPPN PLIPTGTNDS NGGRIIDRLF AGLMSYDAVG KPSLEVAQSI ESADNVNYRI TVKPGWKFTD GSPVTAHSF VDAWNYGALS TNAQLQQHFF SPIEGFDDVA GAPGDKSRTT MSGLRVVNDL EFTVRLKAPT IDFTLRLGHS S FYPLPDSA FRDMAAFGRN PIGNGPYKLA DGPAGPAWEH NVRIDLVPNP DYHGNRKPRN KGLRFEFYAN LDTAYADLLS GN LDVLDTI PPSALTVYQR DLGDHATSGP AAINQTLDTP LRLPHFGGEE GRLRRLALSA AINRPQICQQ IFAGTRSPAR DFT ARSLPG FDPNLPGNEV LDYDPQRARR LWAQADAISP WSGRYAIAYN ADAGHRDWVD AVANSIKNVL GIDAVAAPQP TFAG FRTQI TNRAIDSAFR AGWRGAYPSM IEFLAPLFTA GAGSNDVGYI NPEFDAALAA AEAAPTLTES HELVNDAQRI LFHDM PVVP LWDYISVVGW SSQVSNVTVT WNGLPDYENI VKA UniProtKB: Probable periplasmic dipeptide-binding lipoprotein DppA |
-Macromolecule #5: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 2 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.4000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)