[English] 日本語
 Yorodumi
Yorodumi- EMDB-38244: Open state of central tail fiber of bacteriophage lambda upon bin... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Open state of central tail fiber of bacteriophage lambda upon binding to LamB | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Bacteriophage / caudovirales / siphoviridae / phage lambda / host recognition / LamB / cryo-EM / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont genome ejection through host cell envelope, long flexible tail mechanism / viral tail assembly / virus tail / entry receptor-mediated virion attachment to host cell / host cell cytoplasm / receptor-mediated virion attachment to host cell / virion attachment to host cell Similarity search - Function | |||||||||
| Biological species |  Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.57 Å | |||||||||
 Authors Authors | Ge XF / Wang JW | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural mechanism of bacteriophage lambda tail's interaction with the bacterial receptor. Authors: Xiaofei Ge / Jiawei Wang /  Abstract: Bacteriophage infection, a pivotal process in microbiology, initiates with the phage's tail recognizing and binding to the bacterial cell surface, which then mediates the injection of viral DNA. ...Bacteriophage infection, a pivotal process in microbiology, initiates with the phage's tail recognizing and binding to the bacterial cell surface, which then mediates the injection of viral DNA. Although comprehensive studies on the interaction between bacteriophage lambda and its outer membrane receptor, LamB, have provided rich information about the system's biochemical properties, the precise molecular mechanism remains undetermined. This study revealed the high-resolution cryo-electron microscopy (cryo-EM) structures of the bacteriophage lambda tail complexed with its irreversible Shigella sonnei 3070 LamB receptor and the closed central tail fiber. These structures reveal the complex processes that trigger infection and demonstrate a substantial conformational change in the phage lambda tail tip upon LamB binding. Providing detailed structures of bacteriophage lambda infection initiation, this study contributes to the expanding knowledge of lambda-bacterial interaction, which holds significance in the fields of microbiology and therapeutic development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38244.map.gz emd_38244.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38244-v30.xml emd-38244-v30.xml emd-38244.xml emd-38244.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_38244_fsc.xml emd_38244_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_38244.png emd_38244.png | 172.5 KB | ||
| Filedesc metadata |  emd-38244.cif.gz emd-38244.cif.gz | 6.4 KB | ||
| Others |  emd_38244_half_map_1.map.gz emd_38244_half_map_1.map.gz emd_38244_half_map_2.map.gz emd_38244_half_map_2.map.gz | 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38244 http://ftp.pdbj.org/pub/emdb/structures/EMD-38244 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38244 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38244 | HTTPS FTP |
-Related structure data
| Related structure data |  8xciMC  8xcgC  8xcjC  8xckC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_38244.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38244.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0742 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_38244_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
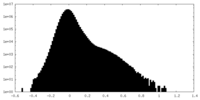
| Density Histograms |
-Half map: #1
| File | emd_38244_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Bacteriophage lambda tail with LamB
| Entire | Name: Bacteriophage lambda tail with LamB |
|---|---|
| Components |
|
-Supramolecule #1: Bacteriophage lambda tail with LamB
| Supramolecule | Name: Bacteriophage lambda tail with LamB / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) |
-Macromolecule #1: Tip attachment protein J
| Macromolecule | Name: Tip attachment protein J / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) |
| Molecular weight | Theoretical: 124.550625 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGKGSSKGHT PREAKDNLKS TQLLSVIDAI SEGPIEGPVD GLKSVLLNST PVLDTEGNTN ISGVTVVFRA GEQEQTPPEG FESSGSETV LGTEVKYDTP ITRTITSANI DRLRFTFGVQ ALVETTSKGD RNPSEVRLLV QIQRNGGWVT EKDITIKGKT T SQYLASVV ...String: MGKGSSKGHT PREAKDNLKS TQLLSVIDAI SEGPIEGPVD GLKSVLLNST PVLDTEGNTN ISGVTVVFRA GEQEQTPPEG FESSGSETV LGTEVKYDTP ITRTITSANI DRLRFTFGVQ ALVETTSKGD RNPSEVRLLV QIQRNGGWVT EKDITIKGKT T SQYLASVV MGNLPPRPFN IRMRRMTPDS TTDQLQNKTL WSSYTEIIDV KQCYPNTALV GVQVDSEQFG SQQVSRNYHL RG RILQVPS NYNPQTRQYS GIWDGTFKPA YSNNMAWCLW DMLTHPRYGM GKRLGAADVD KWALYVIGQY CDQSVPDGFG GTE PRITCN AYLTTQRKAW DVLSDFCSAM RCMPVWNGQT LTFVQDRPSD KTWTYNRSNV VMPDDGAPFR YSFSALKDRH NAVE VNWID PNNGWETATE LVEDTQAIAR YGRNVTKMDA FGCTSRGQAH RAGLWLIKTE LLETQTVDFS VGAEGLRHVP GDVIE ICDD DYAGISTGGR VLAVNSQTRT LTLDREITLP SSGTALISLV DGSGNPVSVE VQSVTDGVKV KVSRVPDGVA EYSVWE LKL PTLRQRLFRC VSIRENDDGT YAITAVQHVP EKEAIVDNGA HFDGEQSGTV NGVTPPAVQH LTAEVTADSG EYQVLAR WD TPKVVKGVSF LLRLTVTADD GSERLVSTAR TTETTYRFTQ LALGNYRLTV RAVNAWGQQG DPASVSFRIA APAAPSRI E LTPGYFQITA TPHLAVYDPT VQFEFWFSEK QIADIRQVET STRYLGTALY WIAASINIKP GHDYYFYIRS VNTVGKSAF VEAVGRASDD AEGYLDFFKG KITESHLGKE LLEKVELTED NASRLEEFSK EWKDASDKWN AMWAVKIEQT KDGKHYVAGI GLSMEDTEE GKLSQFLVAA NRIAFIDPAN GNETPMFVAQ GNQIFMNDVF LKRLTAPTIT SGGNPPAFSL TPDGKLTAKN A DISGSVNA NSGTLSNVTI AENCTINGTL RAEKIVGDIV KAASAAFPRQ RESSVDWPSG TRTVTVTDDH PFDRQIVVLP LT FRGSKRT VSGRTTYSMC YLKVLMNGAV IYDGAANEAV QVFSRIVDMP AGRGNVILTF TLTSTRHSAD IPPYTFASDV QVM VIKKQA LGISVV UniProtKB: Tip attachment protein J |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)