+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | structure of AtHKT1;1 in KCl at 2.8 Angstroms resolution | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HKT / ion selectivity / salt tolerance / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpotassium ion transmembrane transporter activity / response to osmotic stress / sodium ion transport / response to salt stress / potassium ion transport / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Wang J / Su N / Guo J | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Plant / Year: 2024 Journal: Mol Plant / Year: 2024Title: Structures and ion transport mechanisms of plant high-affinity potassium transporters. Authors: Jiangqin Wang / Yanping Luo / Fan Ye / Zhong Jie Ding / Shao Jian Zheng / Shuai Qiao / Yong Wang / Jiangtao Guo / Wei Yang / Nannan Su /  Abstract: Plant high-affinity K transporters (HKTs) mediate Na and K uptake, maintain Na/K homeostasis, and therefore play crucial roles in plant salt tolerance. In this study, we present cryoelectron ...Plant high-affinity K transporters (HKTs) mediate Na and K uptake, maintain Na/K homeostasis, and therefore play crucial roles in plant salt tolerance. In this study, we present cryoelectron microscopy structures of HKTs from two classes, class I HKT1;1 from Arabidopsis thaliana (AtHKT1;1) and class II HKT2;1 from Triticum aestivum (TaHKT2;1), in both Na- and K-bound states at 2.6- to 3.0-Å resolutions. Both AtHKT1;1 and TaHKT2;1 function as homodimers. Each HKT subunit consists of four tandem domain units (D1-D4) with a repeated K-channel-like M-P-M topology. In each subunit, D1-D4 assemble into an ion conduction pore with a pseudo-four-fold symmetry. Although both TaHKT2;1 and AtHKT1;1 have only one putative Na ion bound in the selectivity filter with a similar coordination pattern, the two HKTs display different K binding modes in the filter. TaHKT2;1 has three K ions bound in the selectivity filter, but AtHKT1;1 has only two K ions bound in the filter, which has a narrowed external entrance due to the presence of a Ser residue in the first filter motif. These structures, along with computational, mutational, and electrophysiological analyses, enable us to pinpoint key residues that are critical for the ion selectivity of HKTs. The findings provide new insights into the ion selectivity and ion transport mechanisms of plant HKTs and improve our understanding about how HKTs mediate plant salt tolerance and enhance crop growth. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37377.map.gz emd_37377.map.gz | 28.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37377-v30.xml emd-37377-v30.xml emd-37377.xml emd-37377.xml | 16.9 KB 16.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_37377.png emd_37377.png | 65.3 KB | ||
| Filedesc metadata |  emd-37377.cif.gz emd-37377.cif.gz | 6.2 KB | ||
| Others |  emd_37377_half_map_1.map.gz emd_37377_half_map_1.map.gz emd_37377_half_map_2.map.gz emd_37377_half_map_2.map.gz | 28 MB 28.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37377 http://ftp.pdbj.org/pub/emdb/structures/EMD-37377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37377 | HTTPS FTP |
-Validation report
| Summary document |  emd_37377_validation.pdf.gz emd_37377_validation.pdf.gz | 899.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_37377_full_validation.pdf.gz emd_37377_full_validation.pdf.gz | 898.9 KB | Display | |
| Data in XML |  emd_37377_validation.xml.gz emd_37377_validation.xml.gz | 10.9 KB | Display | |
| Data in CIF |  emd_37377_validation.cif.gz emd_37377_validation.cif.gz | 12.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37377 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37377 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37377 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37377 | HTTPS FTP |
-Related structure data
| Related structure data |  8w9oMC  8w9nC  8w9tC  8w9vC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_37377.map.gz / Format: CCP4 / Size: 35.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37377.map.gz / Format: CCP4 / Size: 35.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.93 Å | ||||||||||||||||||||||||||||||||||||
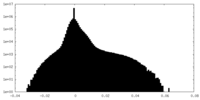
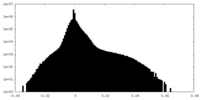
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_37377_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
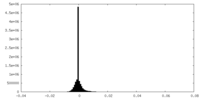
| Density Histograms |
-Half map: #2
| File | emd_37377_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
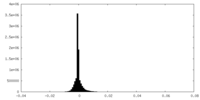
| Density Histograms |
- Sample components
Sample components
-Entire : structure of AtHKT1;1 in KCl at 2.8 Angstroms resolution
| Entire | Name: structure of AtHKT1;1 in KCl at 2.8 Angstroms resolution |
|---|---|
| Components |
|
-Supramolecule #1: structure of AtHKT1;1 in KCl at 2.8 Angstroms resolution
| Supramolecule | Name: structure of AtHKT1;1 in KCl at 2.8 Angstroms resolution type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Sodium transporter HKT1
| Macromolecule | Name: Sodium transporter HKT1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 57.504965 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDRVVAKIAK IRSQLTKLRS LFFLYFIYFL FFSFLGFLAL KITKPRTTSR PHDFDLFFTS VSAITVSSMS TVDMEVFSNT QLIFLTILM FLGGEIFTSF LNLYVSYFTK FVFPHNKIRH ILGSYNSDSS IEDRCDVETV TDYREGLIKI DERASKCLYS V VLSYHLVT ...String: MDRVVAKIAK IRSQLTKLRS LFFLYFIYFL FFSFLGFLAL KITKPRTTSR PHDFDLFFTS VSAITVSSMS TVDMEVFSNT QLIFLTILM FLGGEIFTSF LNLYVSYFTK FVFPHNKIRH ILGSYNSDSS IEDRCDVETV TDYREGLIKI DERASKCLYS V VLSYHLVT NLVGSVLLLV YVNFVKTARD VLSSKEISPL TFSVFTTVST FANCGFVPTN ENMIIFRKNS GLIWLLIPQV LM GNTLFPC FLVLLIWGLY KITKRDEYGY ILKNHNKMGY SHLLSVRLCV LLGVTVLGFL IIQLLFFCAF EWTSESLEGM SSY EKLVGS LFQVVNSRHT GETIVDLSTL SPAILVLFIL MMYLPPYTLF MPLTEQKTIE KEGGDDDSEN GKKVKKSGLI VSQL SFLTI CIFLISITER QNLQRDPINF NVLNITLEVI SAYGNVGFTT GYSCERRVDI SDGGCKDASY GFAGRWSPMG KFVLI IVMF YGRFKQFTAK SGRAWILYPS SS UniProtKB: Sodium transporter HKT1 |
-Macromolecule #2: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 2 / Number of copies: 4 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average exposure time: 8.0 sec. / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)