+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Rpd3S complex from budding yeast | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Rpd3S / HDAC / Sin3 / Rpd3 / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnucleosome disassembly/reassembly complex / negative regulation of antisense RNA transcription / Snt2C complex / negative regulation of reciprocal meiotic recombination / negative regulation of silent mating-type cassette heterochromatin formation / Rpd3L complex / protein localization to nucleolar rDNA repeats / Rpd3L-Expanded complex / negative regulation of rDNA heterochromatin formation / Rpd3S complex ...nucleosome disassembly/reassembly complex / negative regulation of antisense RNA transcription / Snt2C complex / negative regulation of reciprocal meiotic recombination / negative regulation of silent mating-type cassette heterochromatin formation / Rpd3L complex / protein localization to nucleolar rDNA repeats / Rpd3L-Expanded complex / negative regulation of rDNA heterochromatin formation / Rpd3S complex / rDNA chromatin condensation / regulation of RNA stability / nucleophagy / HDACs deacetylate histones / DNA replication-dependent chromatin assembly / histone deacetylase / SUMOylation of chromatin organization proteins / regulation of DNA-templated DNA replication initiation / nucleosome disassembly / cellular response to nitrogen starvation / negative regulation of transcription by RNA polymerase I / histone deacetylase activity / NuA4 histone acetyltransferase complex / Sin3-type complex / Estrogen-dependent gene expression / positive regulation of macroautophagy / histone deacetylase complex / histone acetyltransferase complex / methylated histone binding / nuclear periphery / meiotic cell cycle / positive regulation of transcription elongation by RNA polymerase II / transcription elongation by RNA polymerase II / heterochromatin formation / double-strand break repair via nonhomologous end joining / G1/S transition of mitotic cell cycle / transcription corepressor activity / G2/M transition of mitotic cell cycle / nucleosome assembly / cellular response to heat / response to oxidative stress / transcription coactivator activity / cell division / DNA repair / negative regulation of DNA-templated transcription / DNA-templated transcription / regulation of DNA-templated transcription / chromatin / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / identical protein binding / nucleus / metal ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
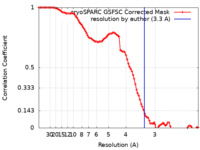
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Wang C / Zhan X | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2024 Journal: Sci Adv / Year: 2024Title: Structures and dynamics of Rpd3S complex bound to nucleosome. Authors: Chengcheng Wang / Chen Chu / Zhouyan Guo / Xiechao Zhan /  Abstract: The Rpd3S complex plays a pivotal role in facilitating local histone deacetylation in the transcribed regions to suppress intragenic transcription initiation. Here, we present the cryo-electron ...The Rpd3S complex plays a pivotal role in facilitating local histone deacetylation in the transcribed regions to suppress intragenic transcription initiation. Here, we present the cryo-electron microscopy structures of the budding yeast Rpd3S complex in both its apo and three nucleosome-bound states at atomic resolutions, revealing the exquisite architecture of Rpd3S to well accommodate a mononucleosome without linker DNA. The Rpd3S core, containing a Sin3 Lobe and two NB modules, is a rigid complex and provides three positive-charged anchors (Sin3_HCR and two Rco1_NIDs) to connect nucleosomal DNA. In three nucleosome-bound states, the Rpd3S core exhibits three distinct orientations relative to the nucleosome, assisting the sector-shaped deacetylase Rpd3 to locate above the SHL5-6, SHL0-1, or SHL2-3, respectively. Our work provides a structural framework that reveals a dynamic working model for the Rpd3S complex to engage diverse deacetylation sites. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37364.map.gz emd_37364.map.gz | 78.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37364-v30.xml emd-37364-v30.xml emd-37364.xml emd-37364.xml | 18.6 KB 18.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_37364_fsc.xml emd_37364_fsc.xml | 9.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_37364.png emd_37364.png | 57.5 KB | ||
| Filedesc metadata |  emd-37364.cif.gz emd-37364.cif.gz | 7.1 KB | ||
| Others |  emd_37364_half_map_1.map.gz emd_37364_half_map_1.map.gz emd_37364_half_map_2.map.gz emd_37364_half_map_2.map.gz | 77.7 MB 77.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37364 http://ftp.pdbj.org/pub/emdb/structures/EMD-37364 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37364 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37364 | HTTPS FTP |
-Validation report
| Summary document |  emd_37364_validation.pdf.gz emd_37364_validation.pdf.gz | 769.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_37364_full_validation.pdf.gz emd_37364_full_validation.pdf.gz | 768.9 KB | Display | |
| Data in XML |  emd_37364_validation.xml.gz emd_37364_validation.xml.gz | 17.3 KB | Display | |
| Data in CIF |  emd_37364_validation.cif.gz emd_37364_validation.cif.gz | 22 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37364 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37364 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37364 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37364 | HTTPS FTP |
-Related structure data
| Related structure data |  8w9cMC  8w9dC  8w9eC  8w9fC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37364.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37364.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.077 Å | ||||||||||||||||||||||||||||||||||||
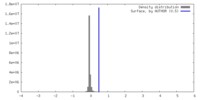
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_37364_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
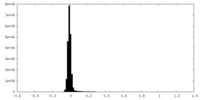
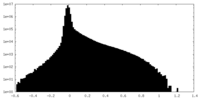
| Density Histograms |
-Half map: #1
| File | emd_37364_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
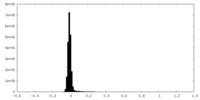
| Density Histograms |
- Sample components
Sample components
-Entire : The Rpd3S complex
| Entire | Name: The Rpd3S complex |
|---|---|
| Components |
|
-Supramolecule #1: The Rpd3S complex
| Supramolecule | Name: The Rpd3S complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Transcriptional regulatory protein SIN3
| Macromolecule | Name: Transcriptional regulatory protein SIN3 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 175.047266 KDa |
| Sequence | String: MSQVWHNSNS QSNDVATSND ATGSNERNEK EPSLQGNKPG FVQQQQRITL PSLSALSTKE EDRRDSNGQQ ALTSHAAHIL GYPPPHSNA MPSIATDSAL KQPHEYHPRP KSSSSSPSIN ASLMNAGPAP LPTVGAASFS LSRFDNPLPI KAPVHTEEPK S YNGLQEEE ...String: MSQVWHNSNS QSNDVATSND ATGSNERNEK EPSLQGNKPG FVQQQQRITL PSLSALSTKE EDRRDSNGQQ ALTSHAAHIL GYPPPHSNA MPSIATDSAL KQPHEYHPRP KSSSSSPSIN ASLMNAGPAP LPTVGAASFS LSRFDNPLPI KAPVHTEEPK S YNGLQEEE KATQRPQDCK EVPAGVQPAD APDPSSNHAD ANDDNNNNEN SHDEDADYRP LNVKDALSYL EQVKFQFSSR PD IYNLFLD IMKDFKSQAI DTPGVIERVS TLFRGYPILI QGFNTFLPQG YRIECSSNPD DPIRVTTPMG TTTVNNNISP SGR GTTDAQ ELGSFPESDG NGVQQPSNVP MVPSSVYQSE QNQDQQQSLP LLATSSGLPS IQQPEMPAHR QIPQSQSLVP QEDA KKNVD VEFSQAISYV NKIKTRFADQ PDIYKHFLEI LQTYQREQKP INEVYAQVTH LFQNAPDLLE DFKKFLPDSS ASANQ QVQH AQQHAQQQHE AQMHAQAQAQ AQAQAQVEQQ KQQQQFLYPA SGYYGHPSNR GIPQQNLPPI GSFSPPTNGS TVHEAY QDQ QHMQPPHFMP LPSIVQHGPN MVHQGIANEN PPLSDLRTSL TEQYAPSSIQ HQQQHPQSIS PIANTQYGDI PVRPEID LD PSIVPVVPEP TEPIENNISL NEEVTFFEKA KRYIGNKHLY TEFLKILNLY SQDILDLDDL VEKVDFYLGS NKELFTWF K NFVGYQEKTK CIENIVHEKH RLDLDLCEAF GPSYKRLPKS DTFMPCSGRD DMCWEVLNDE WVGHPVWASE DSGFIAHRK NQYEETLFKI EEERHEYDFY IESNLRTIQC LETIVNKIEN MTENEKANFK LPPGLGHTSM TIYKKVIRKV YDKERGFEII DALHEHPAV TAPVVLKRLK QKDEEWRRAQ REWNKVWREL EQKVFFKSLD HLGLTFKQAD KKLLTTKQLI SEISSIKVDQ T NKKIHWLT PKPKSQLDFD FPDKNIFYDI LCLADTFITH TTAYSNPDKE RLKDLLKYFI SLFFSISFEK IEESLYSHKQ NV SESSGSD DGSSIASRKR PYQQEMSLLD ILHRSRYQKL KRSNDEDGKV PQLSEPPEEE PNTIEEEELI DEEAKNPWLT GNL VEEANS QGIIQNRSIF NLFANTNIYI FFRHWTTIYE RLLEIKQMNE RVTKEINTRS TVTFAKDLDL LSSQLSEMGL DFVG EDAYK QVLRLSRRLI NGDLEHQWFE ESLRQAYNNK AFKLYTIDKV TQSLVKHAHT LMTDAKTAEI MALFVKDRNA STTSA KDQI IYRLQVRSHM SNTENMFRIE FDKRTLHVSI QYIALDDLTL KEPKADEDKW KYYVTSYALP HPTEGIPHEK LKIPFL ERL IEFGQDIDGT EVDEEFSPEG ISVSTLKIKI QPITYQLHIE NGSYDVFTRK ATNKYPTIAN DNTQKGMVSQ KKELISK FL DCAVGLRNNL DEAQKLSMQK KWENLKDSIA KTSAGNQGIE SETEKGKITK QEQSDNLDSS TASVLPASIT TVPQDDNI E TTGNTESSDK GAKIQ UniProtKB: Transcriptional regulatory protein SIN3 |
-Macromolecule #2: Transcriptional regulatory protein RCO1
| Macromolecule | Name: Transcriptional regulatory protein RCO1 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 78.951305 KDa |
| Sequence | String: MDTSKKDTTR SPSHSNSSSP SSSSLSSSSS KEKKRPKRLS SQNVNYDLKR RKIITSEGIE RSFKNEHSNL AVEDNIPEEE PKELLEKDS KGNIIKLNEP STISEDSKVS VTGLPLNKGP SEKIKRESLW NYRKNLGGQS NNSEMTLVPS KRFTQVPKNF Q DLNRNDLK ...String: MDTSKKDTTR SPSHSNSSSP SSSSLSSSSS KEKKRPKRLS SQNVNYDLKR RKIITSEGIE RSFKNEHSNL AVEDNIPEEE PKELLEKDS KGNIIKLNEP STISEDSKVS VTGLPLNKGP SEKIKRESLW NYRKNLGGQS NNSEMTLVPS KRFTQVPKNF Q DLNRNDLK TFLTENMTEE SNIRSTIGWN GDIINRTRDR EPESDRDNKK LSNIRTKIIL STNATYDSKS KLFGQNSIKS TS NASEKIF RDKNNSTIDF ENEDFCSACN QSGSFLCCDT CPKSFHFLCL DPPIDPNNLP KGDWHCNECK FKIFINNSMA TLK KIESNF IKQNNNVKIF AKLLFNIDSH NPKQFQLPNY IKETFPAVKT GSRGQYSDEN DKIPLTDRQL FNTSYGQSIT KLDS YNPDT HIDSNSGKFL ICYKCNQTRL GSWSHPENSR LIMTCDYCQT PWHLDCVPRA SFKNLGSKWK CPLHSPTKVY KKIHH CQED NSVNYKVWKK QRLINKKNQL YYEPLQKIGY QNNGNIQIIP TTSHTDYDFN QDFKITQIDE NSIKYDFFDK IYKSKM VQK RKLFQFQESL IDKLVSNGSQ NGNSEDNMVK DIASLIYFQV SNNDKSSNNK SASKSNNLRK LWDLKELTNV VVPNELD SI QFNDFSSDEI KHLLYLKKII ESKPKEELLK FLNIENPENQ SE UniProtKB: Transcriptional regulatory protein RCO1 |
-Macromolecule #3: Histone deacetylase RPD3
| Macromolecule | Name: Histone deacetylase RPD3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 48.961957 KDa |
| Sequence | String: MVYEATPFDP ITVKPSDKRR VAYFYDADVG NYAYGAGHPM KPHRIRMAHS LIMNYGLYKK MEIYRAKPAT KQEMCQFHTD EYIDFLSRV TPDNLEMFKR ESVKFNVGDD CPVFDGLYEY CSISGGGSME GAARLNRGKC DVAVNYAGGL HHAKKSEASG F CYLNDIVL ...String: MVYEATPFDP ITVKPSDKRR VAYFYDADVG NYAYGAGHPM KPHRIRMAHS LIMNYGLYKK MEIYRAKPAT KQEMCQFHTD EYIDFLSRV TPDNLEMFKR ESVKFNVGDD CPVFDGLYEY CSISGGGSME GAARLNRGKC DVAVNYAGGL HHAKKSEASG F CYLNDIVL GIIELLRYHP RVLYIDIDVH HGDGVEEAFY TTDRVMTCSF HKYGEFFPGT GELRDIGVGA GKNYAVNVPL RD GIDDATY RSVFEPVIKK IMEWYQPSAV VLQCGGDSLS GDRLGCFNLS MEGHANCVNY VKSFGIPMMV VGGGGYTMRN VAR TWCFET GLLNNVVLDK DLPYNEYYEY YGPDYKLSVR PSNMFNVNTP EYLDKVMTNI FANLENTKYA PSVQLNHTPR DAED LGDVE EDSAEAKDTK GGSQYARDLH VEHDNEFY UniProtKB: Histone deacetylase RPD3 |
-Macromolecule #4: Chromatin modification-related protein EAF3
| Macromolecule | Name: Chromatin modification-related protein EAF3 / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 45.266406 KDa |
| Sequence | String: MVDLEQEFAL GGRCLAFHGP LMYEAKILKI WDPSSKMYTS IPNDKPGGSS QATKEIKPQK LGEDESIPEE IINGKCFFIH YQGWKSSWD EWVGYDRIRA YNEENIAMKK RLANEAKEAK KSLLEQQKKK KLSTSLGGPS NGGKRKGDSR SNASISKSTS Q SFLTSSVS ...String: MVDLEQEFAL GGRCLAFHGP LMYEAKILKI WDPSSKMYTS IPNDKPGGSS QATKEIKPQK LGEDESIPEE IINGKCFFIH YQGWKSSWD EWVGYDRIRA YNEENIAMKK RLANEAKEAK KSLLEQQKKK KLSTSLGGPS NGGKRKGDSR SNASISKSTS Q SFLTSSVS GRKSGRSSAN SLHPGSSLRS SSDQNGNDDR RRSSSLSPNM LHHIAGYPTP KISLQIPIKL KSVLVDDWEY VT KDKKICR LPADVTVEMV LNKYEHEVSQ ELESPGSQSQ LSEYCAGLKL YFDKCLGNML LYRLERLQYD ELLKKSSKDQ KPL VPIRIY GAIHLLRLIS VLPELISSTT MDLQSCQLLI KQTEDFLVWL LMHVDEYFND KDPNRSDDAL YVNTSSQYEG VALG M UniProtKB: Chromatin modification-related protein EAF3 |
-Macromolecule #5: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 5 / Number of copies: 7 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #6: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 6 / Number of copies: 2 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.3000000000000003 µm / Nominal defocus min: 1.7 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)