+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of NaDC1 with Citrate | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Citrate / transporter / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationlow-affinity sodium:dicarboxylate symporter activity / fumarate transmembrane transporter activity / fumarate transport / sodium:dicarboxylate symporter activity / succinate transmembrane transport / Sodium-coupled sulphate, di- and tri-carboxylate transporters / alpha-ketoglutarate transport / alpha-ketoglutarate transmembrane transporter activity / succinate transmembrane transporter activity / cellular response to lithium ion ...low-affinity sodium:dicarboxylate symporter activity / fumarate transmembrane transporter activity / fumarate transport / sodium:dicarboxylate symporter activity / succinate transmembrane transport / Sodium-coupled sulphate, di- and tri-carboxylate transporters / alpha-ketoglutarate transport / alpha-ketoglutarate transmembrane transporter activity / succinate transmembrane transporter activity / cellular response to lithium ion / apical plasma membrane / extracellular exosome / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Chi X / Chen Y / Li Y / Dai L / Zhang Y / Shen Y / Shi T / Yang H / Wang Z / Yan R | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2024 Journal: Sci Adv / Year: 2024Title: Cryo-EM structures of the human NaS1 and NaDC1 transporters revealed the elevator transport and allosteric regulation mechanism. Authors: Ximin Chi / Yiming Chen / Yaning Li / Lu Dai / Yuanyuan Zhang / Yaping Shen / Yun Chen / Tianhao Shi / Haonan Yang / Zilong Wang / Renhong Yan /  Abstract: The solute carrier 13 (SLC13) family comprises electrogenic sodium ion-coupled anion cotransporters, segregating into sodium ion-sulfate cotransporters (NaSs) and sodium ion-di- and-tricarboxylate ...The solute carrier 13 (SLC13) family comprises electrogenic sodium ion-coupled anion cotransporters, segregating into sodium ion-sulfate cotransporters (NaSs) and sodium ion-di- and-tricarboxylate cotransporters (NaDCs). NaS1 and NaDC1 regulate sulfate homeostasis and oxidative metabolism, respectively. NaS1 deficiency affects murine growth and fertility, while NaDC1 affects urinary citrate and calcium nephrolithiasis. Despite their importance, the mechanisms of substrate recognition and transport remain insufficiently characterized. In this study, we determined the cryo-electron microscopy structures of human NaS1, capturing inward-facing and combined inward-facing/outward-facing conformations within a dimer both in apo and sulfate-bound states. In addition, we elucidated NaDC1's outward-facing conformation, encompassing apo, citrate-bound, and -(-amylcinnamoyl) anthranilic acid (ACA) inhibitor-bound states. Structural scrutiny illuminates a detailed elevator mechanism driving conformational changes. Notably, the ACA inhibitor unexpectedly binds primarily anchored by transmembrane 2 (TM2), Loop 10, TM11, and TM6a proximate to the cytosolic membrane. Our findings provide crucial insights into SLC13 transport mechanisms, paving the way for future drug design. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37320.map.gz emd_37320.map.gz | 59.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37320-v30.xml emd-37320-v30.xml emd-37320.xml emd-37320.xml | 15.7 KB 15.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_37320.png emd_37320.png | 61.6 KB | ||
| Filedesc metadata |  emd-37320.cif.gz emd-37320.cif.gz | 5.9 KB | ||
| Others |  emd_37320_half_map_1.map.gz emd_37320_half_map_1.map.gz emd_37320_half_map_2.map.gz emd_37320_half_map_2.map.gz | 47.9 MB 47.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37320 http://ftp.pdbj.org/pub/emdb/structures/EMD-37320 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37320 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37320 | HTTPS FTP |
-Related structure data
| Related structure data |  8w6cMC  8w6dC  8w6gC  8w6hC  8w6nC  8w6oC  8w6tC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_37320.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37320.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_37320_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
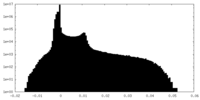
| Projections & Slices |
| ||||||||||||
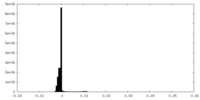
| Density Histograms |
-Half map: #1
| File | emd_37320_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
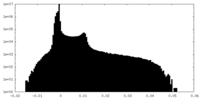
| Projections & Slices |
| ||||||||||||
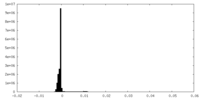
| Density Histograms |
- Sample components
Sample components
-Entire : Structure of NaDC1 in complex with citrate
| Entire | Name: Structure of NaDC1 in complex with citrate |
|---|---|
| Components |
|
-Supramolecule #1: Structure of NaDC1 in complex with citrate
| Supramolecule | Name: Structure of NaDC1 in complex with citrate / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Solute carrier family 13 member 2
| Macromolecule | Name: Solute carrier family 13 member 2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 65.93425 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MADYKDDDDK SGRMATCWQA LWAYRSYLIV FFVPILLLPL PILVPSKEAY CAYAIILMAL FWCTEALPLA VTALFPLILF PMMGIVDAS EVAVEYLKDS NLLFFGGLLV AIAVEHWNLH KRIALRVLLI VGVRPAPLIL GFMLVTAFLS MWISNTATSA M MVPIAHAV ...String: MADYKDDDDK SGRMATCWQA LWAYRSYLIV FFVPILLLPL PILVPSKEAY CAYAIILMAL FWCTEALPLA VTALFPLILF PMMGIVDAS EVAVEYLKDS NLLFFGGLLV AIAVEHWNLH KRIALRVLLI VGVRPAPLIL GFMLVTAFLS MWISNTATSA M MVPIAHAV LDQLHSSQAS SNVEEGSNNP TFELQEPSPQ KEVTKLDNGQ ALPVTSASSE GRAHLSQKHL HLTQCMSLCV CY SASIGGI ATLTGTAPNL VLQGQINSLF PQNGNVVNFA SWFSFAFPTM VILLLLAWLW LQILFLGFNF RKNFGIGEKM QEQ QQAAYC VIQTEHRLLG PMTFAEKAIS ILFVILVLLW FTREPGFFLG WGNLAFPNAK GESMVSDGTV AIFIGIIMFI IPSK FPGLT QDPENPGKLK APLGLLDWKT VNQKMPWNIV LLLGGGYALA KGSERSGLSE WLGNKLTPLQ SVPAPAIAII LSLLV ATFT ECTSNVATTT IFLPILASMA QAICLHPLYV MLPCTLATSL AFMLPVATPP NAIVFSFGDL KVLDMARAGF LLNIIG VLV IALAINSWGI PLFSLHSFPS WAQSNTTAQC LPSLANTTTP SP UniProtKB: Solute carrier family 13 member 2 |
-Macromolecule #2: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 2 / Number of copies: 4 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #3: CHOLESTEROL HEMISUCCINATE
| Macromolecule | Name: CHOLESTEROL HEMISUCCINATE / type: ligand / ID: 3 / Number of copies: 2 / Formula: Y01 |
|---|---|
| Molecular weight | Theoretical: 486.726 Da |
| Chemical component information |  ChemComp-Y01: |
-Macromolecule #4: CITRIC ACID
| Macromolecule | Name: CITRIC ACID / type: ligand / ID: 4 / Number of copies: 2 / Formula: CIT |
|---|---|
| Molecular weight | Theoretical: 192.124 Da |
| Chemical component information |  ChemComp-CIT: |
-Macromolecule #5: 1,2-DIACYL-GLYCEROL-3-SN-PHOSPHATE
| Macromolecule | Name: 1,2-DIACYL-GLYCEROL-3-SN-PHOSPHATE / type: ligand / ID: 5 / Number of copies: 2 / Formula: 3PH |
|---|---|
| Molecular weight | Theoretical: 704.998 Da |
| Chemical component information |  ChemComp-3PH: |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 2 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 1369087 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)