+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | the local map of DNA and Ara-CTP binding site | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | MPXV / complex / RECOMBINATION / REPLICATION / VIRAL PROTEIN | |||||||||
| Biological species |  Monkeypox virus Monkeypox virus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Shen YP / Li YN / Yan RH | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: Structural basis for the inhibition mechanism of the DNA polymerase holoenzyme from mpox virus. Authors: Yaping Shen / Yaning Li / Renhong Yan /  Abstract: There are three key components at the core of the mpox virus (MPXV) DNA polymerase holoenzyme: DNA polymerase F8, processivity factors A22, and the Uracil-DNA glycosylase E4. The holoenzyme is ...There are three key components at the core of the mpox virus (MPXV) DNA polymerase holoenzyme: DNA polymerase F8, processivity factors A22, and the Uracil-DNA glycosylase E4. The holoenzyme is recognized as a vital antiviral target because MPXV replicates in the cytoplasm of host cells. Nucleotide analogs such as cidofovir and cytarabine (Ara-C) have shown potential in curbing MPXV replication and they also display promise against other poxviruses. However, the mechanism behind their inhibitory effects remains unclear. Here, we present the cryo-EM structure of the DNA polymerase holoenzyme F8/A22/E4 bound with its competitive inhibitor Ara-C-derived cytarabine triphosphate (Ara-CTP) at an overall resolution of 3.0 Å and reveal its inhibition mechanism. Ara-CTP functions as a direct chain terminator in proximity to the deoxycytidine triphosphate (dCTP)-binding site. The extra hydrogen bond formed with Asn665 makes it more potent in binding than dCTP. Asn665 is conserved among eukaryotic B-family polymerases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36963.map.gz emd_36963.map.gz | 59.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36963-v30.xml emd-36963-v30.xml emd-36963.xml emd-36963.xml | 13.7 KB 13.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36963.png emd_36963.png | 43.6 KB | ||
| Filedesc metadata |  emd-36963.cif.gz emd-36963.cif.gz | 3.8 KB | ||
| Others |  emd_36963_half_map_1.map.gz emd_36963_half_map_1.map.gz emd_36963_half_map_2.map.gz emd_36963_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36963 http://ftp.pdbj.org/pub/emdb/structures/EMD-36963 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36963 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36963 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36963.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36963.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||
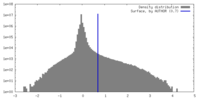
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_36963_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
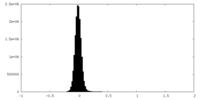
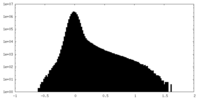
| Density Histograms |
-Half map: #2
| File | emd_36963_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
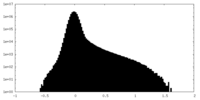
| Density Histograms |
- Sample components
Sample components
-Entire : F8-A22-E4 complex of MPXV in complex with DNA and Ara-CTP
| Entire | Name: F8-A22-E4 complex of MPXV in complex with DNA and Ara-CTP |
|---|---|
| Components |
|
-Supramolecule #1: F8-A22-E4 complex of MPXV in complex with DNA and Ara-CTP
| Supramolecule | Name: F8-A22-E4 complex of MPXV in complex with DNA and Ara-CTP type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Monkeypox virus Monkeypox virus |
-Supramolecule #2: F8-A22-E4 complex of MPXV
| Supramolecule | Name: F8-A22-E4 complex of MPXV / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #3: DNA
| Supramolecule | Name: DNA / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #4-#5 |
|---|
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.4000000000000001 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 4) / Number images used: 266138 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)