[English] 日本語
 Yorodumi
Yorodumi- EMDB-3696: Cryo-EM structure of RNA polymerase-sigma54 holoenzyme with promo... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3696 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
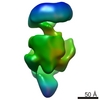
| Title | Cryo-EM structure of RNA polymerase-sigma54 holoenzyme with promoter DNA and transcription activator PspF intermedate complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transcription initiation / transcription intermediate complex / RNA polymerase / sigma54 / transcription | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of cellular response to stress / nitrogen fixation / DNA-binding transcription activator activity / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / regulation of DNA-templated transcription initiation / phosphorelay signal transduction system / sigma factor activity / bacterial-type flagellum assembly ...regulation of cellular response to stress / nitrogen fixation / DNA-binding transcription activator activity / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / regulation of DNA-templated transcription initiation / phosphorelay signal transduction system / sigma factor activity / bacterial-type flagellum assembly / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex / bacterial-type flagellum-dependent cell motility / nitrate assimilation / cis-regulatory region sequence-specific DNA binding / nucleotidyltransferase activity / regulation of DNA-templated transcription elongation / transcription elongation factor complex / transcription antitermination / DNA-directed RNA polymerase complex / cell motility / DNA-templated transcription initiation / protein-DNA complex / ribonucleoside binding / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / response to heat / protein-containing complex assembly / transcription regulator complex / sequence-specific DNA binding / intracellular iron ion homeostasis / protein dimerization activity / transcription cis-regulatory region binding / response to antibiotic / negative regulation of DNA-templated transcription / regulation of DNA-templated transcription / DNA-templated transcription / positive regulation of DNA-templated transcription / magnesium ion binding / ATP hydrolysis activity / DNA binding / zinc ion binding / ATP binding / identical protein binding / membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |   Klebsiella pneumoniae (bacteria) Klebsiella pneumoniae (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.8 Å | |||||||||
 Authors Authors | Glyde R | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2017 Journal: Mol Cell / Year: 2017Title: Structures of RNA Polymerase Closed and Intermediate Complexes Reveal Mechanisms of DNA Opening and Transcription Initiation. Authors: Robert Glyde / Fuzhou Ye / Vidya Chandran Darbari / Nan Zhang / Martin Buck / Xiaodong Zhang /  Abstract: Gene transcription is carried out by RNA polymerases (RNAPs). For transcription to occur, the closed promoter complex (RPc), where DNA is double stranded, must isomerize into an open promoter complex ...Gene transcription is carried out by RNA polymerases (RNAPs). For transcription to occur, the closed promoter complex (RPc), where DNA is double stranded, must isomerize into an open promoter complex (RPo), where the DNA is melted out into a transcription bubble and the single-stranded template DNA is delivered to the RNAP active site. Using a bacterial RNAP containing the alternative σ factor and cryoelectron microscopy, we determined structures of RPc and the activator-bound intermediate complex en route to RPo at 3.8 and 5.8 Å. Our structures show how RNAP-σ interacts with promoter DNA to initiate the DNA distortions required for transcription bubble formation, and how the activator interacts with RPc, leading to significant conformational changes in RNAP and σ that promote RPo formation. We propose that DNA melting is an active process initiated in RPc and that the RNAP conformations of intermediates are significantly different from that of RPc and RPo. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3696.map.gz emd_3696.map.gz | 7.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3696-v30.xml emd-3696-v30.xml emd-3696.xml emd-3696.xml | 30.3 KB 30.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_3696.png emd_3696.png | 70.2 KB | ||
| Filedesc metadata |  emd-3696.cif.gz emd-3696.cif.gz | 9.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3696 http://ftp.pdbj.org/pub/emdb/structures/EMD-3696 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3696 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3696 | HTTPS FTP |
-Related structure data
| Related structure data |  5nssMC  3695C  3697C  5nsrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3696.map.gz / Format: CCP4 / Size: 76.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3696.map.gz / Format: CCP4 / Size: 76.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Cryo-EM structure of RNA polymerase -sigma54 holoenzyme with prom...
+Supramolecule #1: Cryo-EM structure of RNA polymerase -sigma54 holoenzyme with prom...
+Supramolecule #2: Escherichia coli core RNA polymerase
+Supramolecule #3: PspF transcriptional activator
+Supramolecule #4: Sigma-54 transcription initiation factor
+Supramolecule #5: Sigma-54 promoter DNA
+Macromolecule #1: DNA-directed RNA polymerase subunit alpha
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase subunit beta',DNA-directed RNA polyme...
+Macromolecule #4: DNA-directed RNA polymerase subunit omega
+Macromolecule #5: Psp operon transcriptional activator
+Macromolecule #7: RNA polymerase sigma-54 factor,RNA polymerase sigma-54 factor,RNA...
+Macromolecule #6: Non-template promoter DNA
+Macromolecule #8: Template promoter DNA
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 1.25 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)