+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Dimer structure of procaryotic Ago | |||||||||
 Map data Map data | combined focused map, sharpened with deepemhancer | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ago / DNA/RNA / DNA BINDING PROTEIN / CELL INVASION / DNA-RNA-CELL INVASION complex | |||||||||
| Function / homology | SIR2-like domain / DHS-like NAD/FAD-binding domain superfamily / Ribonuclease H superfamily / nucleic acid binding / Ribonuclease H-like superfamily / Piwi domain protein / Sir2 superfamily protein Function and homology information Function and homology information | |||||||||
| Biological species |  Geobacter sulfurreducens (bacteria) / synthetic construct (others) Geobacter sulfurreducens (bacteria) / synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Gao X / Sun D / Cui S / Wang Y | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2024 Journal: Cell Rep / Year: 2024Title: Nucleic acid-induced NADase activation of a short Sir2-associated prokaryotic Argonaute system. Authors: Dapeng Sun / Kaixiang Zhu / Linyue Wang / Zhixia Mu / Kang Wu / Lei Hua / Bo Qin / Xiaopan Gao / Yumei Wang / Sheng Cui /  Abstract: Inhibition of nucleic acid targets is mediated by Argonaute (Ago) proteins guided by RNA or DNA. Although the mechanisms underpinning the functions of eukaryotic and "long" prokaryotic Ago proteins ...Inhibition of nucleic acid targets is mediated by Argonaute (Ago) proteins guided by RNA or DNA. Although the mechanisms underpinning the functions of eukaryotic and "long" prokaryotic Ago proteins (pAgos) are well understood, those for short pAgos remain enigmatic. Here, we determine two cryoelectron microscopy structures of short pAgos in association with the NADase-domain-containing protein Sir2-APAZ from Geobacter sulfurreducens (GsSir2/Ago): the guide RNA-target DNA-loaded GsSir2/Ago quaternary complex (2.58 Å) and the dimer of the quaternary complex (2.93Å). These structures show that the nucleic acid binding causes profound conformational changes that result in disorder or partial dissociation of the Sir2 domain, suggesting that it adopts a NADase-active conformation. Subsequently, two RNA-/DNA-loaded GsSir2/Ago complexes form a dimer through their MID domains, further enhancing NADase activity through synergistic effects. The findings provide a structural basis for short-pAgo-mediated defense against invading nucleic acids. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36946.map.gz emd_36946.map.gz | 57.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36946-v30.xml emd-36946-v30.xml emd-36946.xml emd-36946.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36946.png emd_36946.png | 42.8 KB | ||
| Masks |  emd_36946_msk_1.map emd_36946_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-36946.cif.gz emd-36946.cif.gz | 7 KB | ||
| Others |  emd_36946_half_map_1.map.gz emd_36946_half_map_1.map.gz emd_36946_half_map_2.map.gz emd_36946_half_map_2.map.gz | 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36946 http://ftp.pdbj.org/pub/emdb/structures/EMD-36946 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36946 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36946 | HTTPS FTP |
-Related structure data
| Related structure data |  8k87MC  8k88C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36946.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36946.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | combined focused map, sharpened with deepemhancer | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.009 Å | ||||||||||||||||||||||||||||||||||||
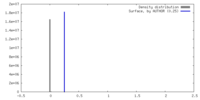
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_36946_msk_1.map emd_36946_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
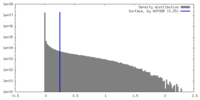
| Density Histograms |
-Half map: #2
| File | emd_36946_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
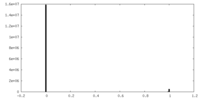
| Density Histograms |
-Half map: #1
| File | emd_36946_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
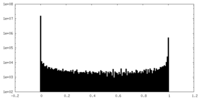
| Density Histograms |
- Sample components
Sample components
-Entire : dimmeric GsSirt2/ago-gRAN-tDNA complex
| Entire | Name: dimmeric GsSirt2/ago-gRAN-tDNA complex |
|---|---|
| Components |
|
-Supramolecule #1: dimmeric GsSirt2/ago-gRAN-tDNA complex
| Supramolecule | Name: dimmeric GsSirt2/ago-gRAN-tDNA complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Geobacter sulfurreducens (bacteria) Geobacter sulfurreducens (bacteria) |
-Macromolecule #1: DNA (41-mer)
| Macromolecule | Name: DNA (41-mer) / type: dna / ID: 1 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 12.525115 KDa |
| Sequence | String: (DC)(DG)(DA)(DA)(DA)(DC)(DA)(DA)(DC)(DA) (DC)(DA)(DG)(DC)(DT)(DC)(DT)(DA)(DA)(DC) (DC)(DG)(DA)(DC)(DG)(DT)(DC)(DT)(DA) (DA)(DG)(DA)(DA)(DA)(DC)(DC)(DA)(DT)(DT) (DA) (DT) |
-Macromolecule #2: RNA/DNA (21-mer)
| Macromolecule | Name: RNA/DNA (21-mer) / type: other / ID: 2 / Number of copies: 2 Classification: polydeoxyribonucleotide/polyribonucleotide hybrid |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 6.675973 KDa |
| Sequence | String: AUAAUGGUUU CUUAGACGUC (DG) |
-Macromolecule #3: Piwi domain protein
| Macromolecule | Name: Piwi domain protein / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Geobacter sulfurreducens (bacteria) Geobacter sulfurreducens (bacteria) |
| Molecular weight | Theoretical: 53.325566 KDa |
| Recombinant expression | Organism: Expression vector pET-mod (others) |
| Sequence | String: MADNLSQLAA HSTIPEPLLL FKDNRTDTHP LRGLSQYGPY SACFNLPGQV RLAYLAPTEH MRKLDAIVRE LQNPATPKEA TNYYVEYGG FEKVFKVPLV MPQEHLRCLA LDECHGVAAN GNGLALADKI VQSMSGLFRQ KHAFDVLLVY LPASWKKCFE Y DGFDLHDR ...String: MADNLSQLAA HSTIPEPLLL FKDNRTDTHP LRGLSQYGPY SACFNLPGQV RLAYLAPTEH MRKLDAIVRE LQNPATPKEA TNYYVEYGG FEKVFKVPLV MPQEHLRCLA LDECHGVAAN GNGLALADKI VQSMSGLFRQ KHAFDVLLVY LPASWKKCFE Y DGFDLHDR IKAKVAPLNL PIQIINDTAL TRQCRANVMW GVSVALYAKA GGIPWKLADW DKDEAYIGLS YAIKKNAEGQ EY TTCCSQV FDPDGTGFEF VAYDTREFIT DRKGNPYLSY QEMQSVLSKS LHLYQSSHNG RMPRKIFIHK TTHFTEDEIQ GAF DSFSSS TEIELVQIIQ STNWYGLKVD GKKGDKPVAP ASYPVDRGLY QPLTESECLL WTQGSVMGVN QQNPGQPVFK EAAL TPLPN PIMLRRFSGN GGWHATCSSI LALTKVDWNN NTLYKKLPVT LVYSQVFADV VKQTPEIVNE IYDYRFFM UniProtKB: Piwi domain protein |
-Macromolecule #4: Sir2 superfamily protein
| Macromolecule | Name: Sir2 superfamily protein / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Geobacter sulfurreducens (bacteria) Geobacter sulfurreducens (bacteria) |
| Molecular weight | Theoretical: 66.645922 KDa |
| Recombinant expression | Organism: Expression vector pET-mod (others) |
| Sequence | String: MDVLTDNEFY QHYLQNSQHM MWFLGAGTSR SAGLPTASDI IWDLKHRYYC LHENQDYQKH DINNHAIKSK IQSYMDSKGF PLQWSPEEY SFYFELVFRD DYEAQRKYLL EALASRKVSL NIGHRVLAAL LEMNQTKVVF TTNFDDVIET AFSDISGKHL S VYHLEGSY ...String: MDVLTDNEFY QHYLQNSQHM MWFLGAGTSR SAGLPTASDI IWDLKHRYYC LHENQDYQKH DINNHAIKSK IQSYMDSKGF PLQWSPEEY SFYFELVFRD DYEAQRKYLL EALASRKVSL NIGHRVLAAL LEMNQTKVVF TTNFDDVIET AFSDISGKHL S VYHLEGSY AALSALNTEA FPIYAKIHGD FRYQKIKNLT PDLQTNDREI HKCFLAAAIR FGLVVSGYSG RDENVMTMLR AA IDQNNAF PHGLYWTVPS ISKSEPAVQD LITYAQGKGV RAYLVETGTF DEMLSKIWRQ VKDKPAAIDA KVRTARVCPV SIP LPGPGK SFPALRTNAL PVVTQSIRCG VVTLASPITF SELKERISQK SPKALLTYTE KVLFLGGEPE IRKIFSNDEI NSIG QYYID EIAQSVAAST FLKSFVEEAI LTALLREKPI LHRVRHRTHY AVIPNASAKD DRFLDLRKAV GFKGDLGYIT GNVTN AKEL SWAEAVSIRL EERGGKLWIM LKPEIWIKPL DRREEATDFI RSRRRYRFNQ CSYQILDAWI KILFGSIGGG GTVNIS CFP DAEFKAEFEI GTRTAFSLGV GYGR UniProtKB: Sir2 superfamily protein |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)