+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | AHS-CSF domains of phage lambda tail | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Bacteriophage / caudovirales / siphoviridae / tail complex / delivery device / phage lambda / cryo-EM / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont genome ejection through host cell envelope, long flexible tail mechanism / viral tail assembly / virus tail / host cell cytoplasm / entry receptor-mediated virion attachment to host cell / receptor-mediated virion attachment to host cell / virion attachment to host cell Similarity search - Function | |||||||||
| Biological species |  Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) | |||||||||
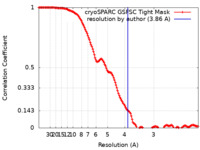
| Method | single particle reconstruction / cryo EM / Resolution: 3.86 Å | |||||||||
 Authors Authors | Wang J | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: Architecture of the bacteriophage lambda tail. Authors: Chang Wang / Jinsong Duan / Zhiwei Gu / Xiaofei Ge / Jianwei Zeng / Jiawei Wang /  Abstract: Bacteriophage lambda has a double-stranded DNA genome and a long, flexible, non-contractile tail encoded by a contiguous block of 11 genes downstream of the head genes. The tail allows host ...Bacteriophage lambda has a double-stranded DNA genome and a long, flexible, non-contractile tail encoded by a contiguous block of 11 genes downstream of the head genes. The tail allows host recognition and delivery of viral DNA from the head shell to the cytoplasm of the infected cell. Here, we present a high-resolution structure of the tail complex of bacteriophage lambda determined by cryoelectron microscopy. Most component proteins of the lambda tail were determined at the atomic scale. The structure sheds light on the molecular organization of the extensively studied tail of bacteriophage lambda. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36677.map.gz emd_36677.map.gz | 229.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36677-v30.xml emd-36677-v30.xml emd-36677.xml emd-36677.xml | 17.7 KB 17.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36677_fsc.xml emd_36677_fsc.xml | 13.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_36677.png emd_36677.png | 50.2 KB | ||
| Filedesc metadata |  emd-36677.cif.gz emd-36677.cif.gz | 5.7 KB | ||
| Others |  emd_36677_half_map_1.map.gz emd_36677_half_map_1.map.gz emd_36677_half_map_2.map.gz emd_36677_half_map_2.map.gz | 226.6 MB 226.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36677 http://ftp.pdbj.org/pub/emdb/structures/EMD-36677 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36677 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36677 | HTTPS FTP |
-Validation report
| Summary document |  emd_36677_validation.pdf.gz emd_36677_validation.pdf.gz | 731.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36677_full_validation.pdf.gz emd_36677_full_validation.pdf.gz | 731.4 KB | Display | |
| Data in XML |  emd_36677_validation.xml.gz emd_36677_validation.xml.gz | 21.6 KB | Display | |
| Data in CIF |  emd_36677_validation.cif.gz emd_36677_validation.cif.gz | 28.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36677 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36677 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36677 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36677 | HTTPS FTP |
-Related structure data
| Related structure data |  8jvmMC  8iydC  8iykC  8iylC  8kgeC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36677.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36677.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0742 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half 1
| File | emd_36677_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half 2
| File | emd_36677_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : bacteriophage lambda tail
| Entire | Name: bacteriophage lambda tail |
|---|---|
| Components |
|
-Supramolecule #1: bacteriophage lambda tail
| Supramolecule | Name: bacteriophage lambda tail / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) |
-Macromolecule #1: Tip attachment protein J
| Macromolecule | Name: Tip attachment protein J / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) |
| Molecular weight | Theoretical: 17.734773 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ELLEKVELTE DNASRLEEFS KEWKDASDKW NAMAAVKAEQ TKDGKHYVAG IGLSMEDTEE GKLSQFLVAA NRIAAIDPAN GNETPMFVA QGNQIFMNDV FLKRLTAPTI TSGGNPPAFS LTPDGKLTAK NADISGSVNA NSGTLSNVTI AENCTINGTL R AEKIVG UniProtKB: Tip attachment protein J |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: NITROGEN |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)