[English] 日本語
 Yorodumi
Yorodumi- EMDB-36668: Cryo-EM structure of the zebrafish P2X4 receptor in complex with BX430 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the zebrafish P2X4 receptor in complex with BX430 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATP / allosteric modulator / channel / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationElevation of cytosolic Ca2+ levels / Platelet homeostasis / purine nucleotide binding / extracellularly ATP-gated monoatomic cation channel activity / purinergic nucleotide receptor activity / transmembrane transporter complex / ATP-gated ion channel activity / ligand-gated monoatomic ion channel activity / CTP binding / response to ATP ...Elevation of cytosolic Ca2+ levels / Platelet homeostasis / purine nucleotide binding / extracellularly ATP-gated monoatomic cation channel activity / purinergic nucleotide receptor activity / transmembrane transporter complex / ATP-gated ion channel activity / ligand-gated monoatomic ion channel activity / CTP binding / response to ATP / monoatomic cation transport / monoatomic ion channel complex / calcium ion transport / postsynapse / lysosomal membrane / ATP binding / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
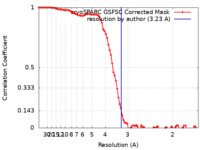
| Method | single particle reconstruction / cryo EM / Resolution: 3.23 Å | |||||||||
 Authors Authors | Hattori M / Shen C | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural insights into the allosteric inhibition of P2X4 receptors. Authors: Cheng Shen / Yuqing Zhang / Wenwen Cui / Yimeng Zhao / Danqi Sheng / Xinyu Teng / Miaoqing Shao / Muneyoshi Ichikawa / Jin Wang / Motoyuki Hattori /  Abstract: P2X receptors are ATP-activated cation channels, and the P2X4 subtype plays important roles in the immune system and the central nervous system, particularly in neuropathic pain. Therefore, P2X4 ...P2X receptors are ATP-activated cation channels, and the P2X4 subtype plays important roles in the immune system and the central nervous system, particularly in neuropathic pain. Therefore, P2X4 receptors are of increasing interest as potential drug targets. Here, we report the cryo-EM structures of the zebrafish P2X4 receptor in complex with two P2X4 subtype-specific antagonists, BX430 and BAY-1797. Both antagonists bind to the same allosteric site located at the subunit interface at the top of the extracellular domain. Structure-based mutational analysis by electrophysiology identified the important residues for the allosteric inhibition of both zebrafish and human P2X4 receptors. Structural comparison revealed the ligand-dependent structural rearrangement of the binding pocket to stabilize the binding of allosteric modulators, which in turn would prevent the structural changes of the extracellular domain associated with channel activation. Furthermore, comparison with the previously reported P2X structures of other subtypes provided mechanistic insights into subtype-specific allosteric inhibition. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36668.map.gz emd_36668.map.gz | 32.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36668-v30.xml emd-36668-v30.xml emd-36668.xml emd-36668.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36668_fsc.xml emd_36668_fsc.xml | 9.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_36668.png emd_36668.png | 44.5 KB | ||
| Filedesc metadata |  emd-36668.cif.gz emd-36668.cif.gz | 6.2 KB | ||
| Others |  emd_36668_half_map_1.map.gz emd_36668_half_map_1.map.gz emd_36668_half_map_2.map.gz emd_36668_half_map_2.map.gz | 59.1 MB 59.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36668 http://ftp.pdbj.org/pub/emdb/structures/EMD-36668 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36668 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36668 | HTTPS FTP |
-Validation report
| Summary document |  emd_36668_validation.pdf.gz emd_36668_validation.pdf.gz | 749.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36668_full_validation.pdf.gz emd_36668_full_validation.pdf.gz | 749.3 KB | Display | |
| Data in XML |  emd_36668_validation.xml.gz emd_36668_validation.xml.gz | 16.3 KB | Display | |
| Data in CIF |  emd_36668_validation.cif.gz emd_36668_validation.cif.gz | 21.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36668 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36668 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36668 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36668 | HTTPS FTP |
-Related structure data
| Related structure data |  8jv5MC  8jv6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36668.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36668.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_36668_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36668_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : P2X4 trimer
| Entire | Name: P2X4 trimer |
|---|---|
| Components |
|
-Supramolecule #1: P2X4 trimer
| Supramolecule | Name: P2X4 trimer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 120 KDa |
-Macromolecule #1: P2X purinoceptor
| Macromolecule | Name: P2X purinoceptor / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.476988 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DSVSQCFFDY YTSKILIIRS KKVGTLNRFT QALVIAYVIG YVCVYNKGYQ DTDTVLSSVT TKVKGIALTN TSELGERIWD VADYIIPPQ EDGSFFVLTN MIITTNQTQS KCAENPTPAS TCTSHRDCKR GFNDARGDGV RTGRCVSYSA SVKTCEVLSW C PLEKIVDP ...String: DSVSQCFFDY YTSKILIIRS KKVGTLNRFT QALVIAYVIG YVCVYNKGYQ DTDTVLSSVT TKVKGIALTN TSELGERIWD VADYIIPPQ EDGSFFVLTN MIITTNQTQS KCAENPTPAS TCTSHRDCKR GFNDARGDGV RTGRCVSYSA SVKTCEVLSW C PLEKIVDP PNPPLLADAE NFTVLIKNNI RYPKFNFNKR NILPNINSSY LTHCVFSRKT DPDCPIFRLG DIVGEAEEDF QI MAVRGGV MGVQIRWDCD LDMPQSWCVP RYTFRRLDNK DPDNNVAPGY NFRFAKYYKN SDGTETRTLI KGYGIRFDVM VFG QAGKFN IIPTLLNIGA GLALLGLVNV ICDWI UniProtKB: P2X purinoceptor 4a |
-Macromolecule #2: 1-[2,6-bis(bromanyl)-4-propan-2-yl-phenyl]-3-pyridin-3-yl-urea
| Macromolecule | Name: 1-[2,6-bis(bromanyl)-4-propan-2-yl-phenyl]-3-pyridin-3-yl-urea type: ligand / ID: 2 / Number of copies: 3 / Formula: P73 |
|---|---|
| Molecular weight | Theoretical: 413.107 Da |
| Chemical component information | 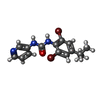 ChemComp-P73: |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 9 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)