[English] 日本語
 Yorodumi
Yorodumi- EMDB-36369: Focused map on the aCTD-AfsR(T337A) region of the Streptomyces co... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Focused map on the aCTD-AfsR(T337A) region of the Streptomyces coelicolor RNAP-promoter open complex with AfsR(T337A) dimer | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA polymerase / SARP regulator / gene regulation | |||||||||
| Biological species |  Streptomyces coelicolor A3(2) (bacteria) Streptomyces coelicolor A3(2) (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.1 Å | |||||||||
 Authors Authors | Wang Y / Zheng J | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: PLoS Biol / Year: 2024 Journal: PLoS Biol / Year: 2024Title: Structural and functional characterization of AfsR, an SARP family transcriptional activator of antibiotic biosynthesis in Streptomyces. Authors: Yiqun Wang / Xu Yang / Feng Yu / Zixin Deng / Shuangjun Lin / Jianting Zheng /  Abstract: Streptomyces antibiotic regulatory proteins (SARPs) are widely distributed activators of antibiotic biosynthesis. Streptomyces coelicolor AfsR is an SARP regulator with an additional nucleotide- ...Streptomyces antibiotic regulatory proteins (SARPs) are widely distributed activators of antibiotic biosynthesis. Streptomyces coelicolor AfsR is an SARP regulator with an additional nucleotide-binding oligomerization domain (NOD) and a tetratricopeptide repeat (TPR) domain. Here, we present cryo-electron microscopy (cryo-EM) structures and in vitro assays to demonstrate how the SARP domain activates transcription and how it is modulated by NOD and TPR domains. The structures of transcription initiation complexes (TICs) show that the SARP domain forms a side-by-side dimer to simultaneously engage the afs box overlapping the -35 element and the σHrdB region 4 (R4), resembling a sigma adaptation mechanism. The SARP extensively interacts with the subunits of the RNA polymerase (RNAP) core enzyme including the β-flap tip helix (FTH), the β' zinc-binding domain (ZBD), and the highly flexible C-terminal domain of the α subunit (αCTD). Transcription assays of full-length AfsR and truncated proteins reveal the inhibitory effect of NOD and TPR on SARP transcription activation, which can be eliminated by ATP binding. In vitro phosphorylation hardly affects transcription activation of AfsR, but counteracts the disinhibition of ATP binding. Overall, our results present a detailed molecular view of how AfsR serves to activate transcription. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36369.map.gz emd_36369.map.gz | 1.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36369-v30.xml emd-36369-v30.xml emd-36369.xml emd-36369.xml | 15.8 KB 15.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36369_fsc.xml emd_36369_fsc.xml | 13.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_36369.png emd_36369.png | 36.3 KB | ||
| Masks |  emd_36369_msk_1.map emd_36369_msk_1.map | 274.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-36369.cif.gz emd-36369.cif.gz | 4.5 KB | ||
| Others |  emd_36369_half_map_1.map.gz emd_36369_half_map_1.map.gz emd_36369_half_map_2.map.gz emd_36369_half_map_2.map.gz | 254.4 MB 254.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36369 http://ftp.pdbj.org/pub/emdb/structures/EMD-36369 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36369 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36369 | HTTPS FTP |
-Validation report
| Summary document |  emd_36369_validation.pdf.gz emd_36369_validation.pdf.gz | 982.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36369_full_validation.pdf.gz emd_36369_full_validation.pdf.gz | 981.9 KB | Display | |
| Data in XML |  emd_36369_validation.xml.gz emd_36369_validation.xml.gz | 22.8 KB | Display | |
| Data in CIF |  emd_36369_validation.cif.gz emd_36369_validation.cif.gz | 29.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36369 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36369 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36369 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36369 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36369.map.gz / Format: CCP4 / Size: 274.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36369.map.gz / Format: CCP4 / Size: 274.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||
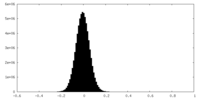
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_36369_msk_1.map emd_36369_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_36369_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36369_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : AfsR(T337A)-dependent transcription activation complex with afsS ...
| Entire | Name: AfsR(T337A)-dependent transcription activation complex with afsS promoter |
|---|---|
| Components |
|
-Supramolecule #1: AfsR(T337A)-dependent transcription activation complex with afsS ...
| Supramolecule | Name: AfsR(T337A)-dependent transcription activation complex with afsS promoter type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#11 |
|---|---|
| Source (natural) | Organism:  Streptomyces coelicolor A3(2) (bacteria) Streptomyces coelicolor A3(2) (bacteria) |
| Molecular weight | Theoretical: 700 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 289 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 77.0 K / Max: 77.0 K |
| Specialist optics | Energy filter - Name: GIF Bioquantum |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|
 Movie
Movie Controller
Controller


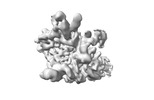





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)