[English] 日本語
 Yorodumi
Yorodumi- EMDB-36201: Human sodium-dependent vitamin C transporter 1 bound to L-ascorbi... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human sodium-dependent vitamin C transporter 1 bound to L-ascorbic acid in an inward-open state | |||||||||
 Map data Map data | FSC-weighted, sharpened and masked map by PostProcess | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Transporter / Membrane protein / Ascorbic acid / Vitamin C / Sodium / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnucleobase transport / L-ascorbate:sodium symporter activity / L-ascorbic acid transmembrane transporter activity / L-ascorbic acid transmembrane transport / nucleobase transmembrane transporter activity / dehydroascorbic acid transmembrane transporter activity / intracellular organelle / sodium ion transmembrane transporter activity / dehydroascorbic acid transport / Vitamin C (ascorbate) metabolism ...nucleobase transport / L-ascorbate:sodium symporter activity / L-ascorbic acid transmembrane transporter activity / L-ascorbic acid transmembrane transport / nucleobase transmembrane transporter activity / dehydroascorbic acid transmembrane transporter activity / intracellular organelle / sodium ion transmembrane transporter activity / dehydroascorbic acid transport / Vitamin C (ascorbate) metabolism / L-ascorbic acid metabolic process / urate transmembrane transporter activity / sodium ion transport / lung development / basal plasma membrane / brain development / response to toxic substance / apical plasma membrane / extracellular exosome / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
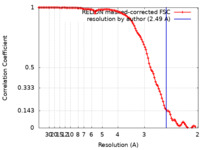
| Method | single particle reconstruction / cryo EM / Resolution: 2.49 Å | |||||||||
 Authors Authors | Kobayashi TA / Kusakizako T / Nureki O | |||||||||
| Funding support |  Japan, 1 items Japan, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Dimeric transport mechanism of human vitamin C transporter SVCT1. Authors: Takaaki A Kobayashi / Hiroto Shimada / Fumiya K Sano / Yuzuru Itoh / Sawako Enoki / Yasushi Okada / Tsukasa Kusakizako / Osamu Nureki /  Abstract: Vitamin C plays important roles as a cofactor in many enzymatic reactions and as an antioxidant against oxidative stress. As some mammals including humans cannot synthesize vitamin C de novo from ...Vitamin C plays important roles as a cofactor in many enzymatic reactions and as an antioxidant against oxidative stress. As some mammals including humans cannot synthesize vitamin C de novo from glucose, its uptake from dietary sources is essential, and is mediated by the sodium-dependent vitamin C transporter 1 (SVCT1). Despite its physiological significance in maintaining vitamin C homeostasis, the structural basis of the substrate transport mechanism remained unclear. Here, we report the cryo-EM structures of human SVCT1 in different states at 2.5-3.5 Å resolutions. The binding manner of vitamin C together with two sodium ions reveals the counter ion-dependent substrate recognition mechanism. Furthermore, comparisons of the inward-open and occluded structures support a transport mechanism combining elevator and distinct rotational motions. Our results demonstrate the molecular mechanism of vitamin C transport with its underlying conformational cycle, potentially leading to future industrial and medical applications. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36201.map.gz emd_36201.map.gz | 2.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36201-v30.xml emd-36201-v30.xml emd-36201.xml emd-36201.xml | 18.9 KB 18.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36201_fsc.xml emd_36201_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_36201.png emd_36201.png | 112.5 KB | ||
| Masks |  emd_36201_msk_1.map emd_36201_msk_1.map | 8 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-36201.cif.gz emd-36201.cif.gz | 6.9 KB | ||
| Others |  emd_36201_half_map_1.map.gz emd_36201_half_map_1.map.gz emd_36201_half_map_2.map.gz emd_36201_half_map_2.map.gz | 6.7 MB 6.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36201 http://ftp.pdbj.org/pub/emdb/structures/EMD-36201 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36201 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36201 | HTTPS FTP |
-Validation report
| Summary document |  emd_36201_validation.pdf.gz emd_36201_validation.pdf.gz | 825.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36201_full_validation.pdf.gz emd_36201_full_validation.pdf.gz | 825.2 KB | Display | |
| Data in XML |  emd_36201_validation.xml.gz emd_36201_validation.xml.gz | 13.4 KB | Display | |
| Data in CIF |  emd_36201_validation.cif.gz emd_36201_validation.cif.gz | 17.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36201 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36201 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36201 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36201 | HTTPS FTP |
-Related structure data
| Related structure data |  8jewMC  8jezC  8jf0C  8jf1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36201.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36201.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | FSC-weighted, sharpened and masked map by PostProcess | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.996 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_36201_msk_1.map emd_36201_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36201_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_36201_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Human SVCT1 dimer in a substrate-bound inward-open state
+Supramolecule #1: Human SVCT1 dimer in a substrate-bound inward-open state
+Macromolecule #1: Solute carrier family 23 member 1
+Macromolecule #2: ASCORBIC ACID
+Macromolecule #3: SODIUM ION
+Macromolecule #4: Lauryl Maltose Neopentyl Glycol
+Macromolecule #5: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
+Macromolecule #6: CHOLESTEROL
+Macromolecule #7: 2-acetamido-2-deoxy-beta-D-glucopyranose
+Macromolecule #8: PALMITIC ACID
+Macromolecule #9: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 25 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 3753 / Average electron dose: 49.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)