+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | DltB tetramer in complex with inhibitor m-AMSA | |||||||||
 Map data Map data | This map is reconstructed from cryo-EM images collected from K3 camera of TITAN KRIOSg3i and is analysised mainly by the cryoSPARC and Relion. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | channel / anti-virulence / MBOAT / DltB / MEMBRANE PROTEIN / MEMBRANE PROTEIN-INHIBITOR complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationlipoteichoic acid biosynthetic process / acyltransferase activity / Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Streptococcus thermophilus (bacteria) / Streptococcus thermophilus (bacteria) /  Streptococcus thermophilus LMG 18311 (bacteria) Streptococcus thermophilus LMG 18311 (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.23 Å | |||||||||
 Authors Authors | Zhang P / Liu Z | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural insights into the transporting and catalyzing mechanism of DltB in LTA D-alanylation. Authors: Pingfeng Zhang / Zheng Liu /  Abstract: DltB, a model member of the Membrane-Bound O-AcylTransferase (MBOAT) superfamily, plays a crucial role in D-alanylation of the lipoteichoic acid (LTA), a significant component of the cell wall of ...DltB, a model member of the Membrane-Bound O-AcylTransferase (MBOAT) superfamily, plays a crucial role in D-alanylation of the lipoteichoic acid (LTA), a significant component of the cell wall of gram-positive bacteria. This process stabilizes the cell wall structure, influences bacterial virulence, and modulates the host immune response. Despite its significance, the role of DltB is not well understood. Through biochemical analysis and cryo-EM imaging, we discover that Streptococcus thermophilus DltB forms a homo-tetramer on the cell membrane. We further visualize DltB in an apo form, in complex with DltC, and in complex with its inhibitor amsacrine (m-AMSA). Each tetramer features a central hole. The C-tunnel of each protomer faces the intratetramer interface and provides access to the periphery membrane. Each protomer binds a DltC without changing the tetrameric organization. A phosphatidylglycerol (PG) molecule in the substrate-binding site may serve as an LTA carrier. The inhibitor m-AMSA bound to the L-tunnel of each protomer blocks the active site. The tetrameric organization of DltB provides a scaffold for catalyzing D-alanyl transfer and regulating the channel opening and closing. Our findings unveil DltB's dual function in the D-alanylation pathway, and provide insight for targeting DltB as a anti-virulence antibiotic. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36192.map.gz emd_36192.map.gz | 117.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36192-v30.xml emd-36192-v30.xml emd-36192.xml emd-36192.xml | 19 KB 19 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36192.png emd_36192.png | 209.5 KB | ||
| Filedesc metadata |  emd-36192.cif.gz emd-36192.cif.gz | 6.9 KB | ||
| Others |  emd_36192_half_map_1.map.gz emd_36192_half_map_1.map.gz emd_36192_half_map_2.map.gz emd_36192_half_map_2.map.gz | 115.8 MB 115.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36192 http://ftp.pdbj.org/pub/emdb/structures/EMD-36192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36192 | HTTPS FTP |
-Validation report
| Summary document |  emd_36192_validation.pdf.gz emd_36192_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36192_full_validation.pdf.gz emd_36192_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_36192_validation.xml.gz emd_36192_validation.xml.gz | 13.9 KB | Display | |
| Data in CIF |  emd_36192_validation.cif.gz emd_36192_validation.cif.gz | 16.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36192 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36192 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36192 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36192 | HTTPS FTP |
-Related structure data
| Related structure data |  8jemMC  8jesC  8jf2C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36192.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36192.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This map is reconstructed from cryo-EM images collected from K3 camera of TITAN KRIOSg3i and is analysised mainly by the cryoSPARC and Relion. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_36192_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36192_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : DltB tetramer in complex with inhibitor m-AMSA.
| Entire | Name: DltB tetramer in complex with inhibitor m-AMSA. |
|---|---|
| Components |
|
-Supramolecule #1: DltB tetramer in complex with inhibitor m-AMSA.
| Supramolecule | Name: DltB tetramer in complex with inhibitor m-AMSA. / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Streptococcus thermophilus (bacteria) / Strain: LMG 18311 Streptococcus thermophilus (bacteria) / Strain: LMG 18311 |
| Molecular weight | Theoretical: 48 kDa/nm |
-Macromolecule #1: Teichoic acid D-alanyltransferase
| Macromolecule | Name: Teichoic acid D-alanyltransferase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO EC number: Transferases; Acyltransferases; Transferring groups other than aminoacyl groups |
|---|---|
| Source (natural) | Organism:  Streptococcus thermophilus LMG 18311 (bacteria) / Strain: LMG 18311 Streptococcus thermophilus LMG 18311 (bacteria) / Strain: LMG 18311 |
| Molecular weight | Theoretical: 51.780027 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH NYDIPTTENL YFQGSMIDFL KQLPHLEPYG NPFYFIYLGI ALLPIFIGLF FKKRFAIYEC LVSITFIVLA LTGTHASQI LALLFYIVWQ IIWVYSYKRY RSQRDNKWVF YLHSFLVVLP LILVKVEPTI NGTQSLLNFL GISYLTFRAV G MIIEMRDG ...String: MGSSHHHHHH NYDIPTTENL YFQGSMIDFL KQLPHLEPYG NPFYFIYLGI ALLPIFIGLF FKKRFAIYEC LVSITFIVLA LTGTHASQI LALLFYIVWQ IIWVYSYKRY RSQRDNKWVF YLHSFLVVLP LILVKVEPTI NGTQSLLNFL GISYLTFRAV G MIIEMRDG VLKEFTLGEF LRFMLFMPTF TSGPIDRFKR FNEDYQSIPN RDELLNMLEQ AVKYIMLGFL YKFVLAQIFG SM LLPPLKA QALSQGGIFN LPTLGVMYVY GFDLFFDFAG YSMFALAVSN LMGIKSPINF DKPFISRDMK EFWNRWHMSL SFW FRDFVF MRLVIVLMRN KVFKNRNTTS NVAYIINMMV MGFWHGITWY YIAYGIFHGI GLVINDAWLR KKKTINKDRK KAGL KPLPE NKWTKALGIF ITFNTVMLSF LIFSGFLNDL WFTKK UniProtKB: Teichoic acid D-alanyltransferase |
-Macromolecule #2: N-[4-(acridin-9-ylamino)-3-methoxyphenyl]methanesulfonamide
| Macromolecule | Name: N-[4-(acridin-9-ylamino)-3-methoxyphenyl]methanesulfonamide type: ligand / ID: 2 / Number of copies: 4 / Formula: ASW |
|---|---|
| Molecular weight | Theoretical: 393.459 Da |
| Chemical component information | 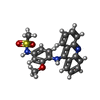 ChemComp-ASW: |
-Macromolecule #3: (1S)-2-{[{[(2R)-2,3-DIHYDROXYPROPYL]OXY}(HYDROXY)PHOSPHORYL]OXY}-...
| Macromolecule | Name: (1S)-2-{[{[(2R)-2,3-DIHYDROXYPROPYL]OXY}(HYDROXY)PHOSPHORYL]OXY}-1-[(PALMITOYLOXY)METHYL]ETHYL STEARATE type: ligand / ID: 3 / Number of copies: 8 / Formula: PGT |
|---|---|
| Molecular weight | Theoretical: 751.023 Da |
| Chemical component information |  ChemComp-PGT: |
-Macromolecule #4: DODECYL-BETA-D-MALTOSIDE
| Macromolecule | Name: DODECYL-BETA-D-MALTOSIDE / type: ligand / ID: 4 / Number of copies: 40 / Formula: LMT |
|---|---|
| Molecular weight | Theoretical: 510.615 Da |
| Chemical component information |  ChemComp-LMT: |
-Macromolecule #5: DIACYL GLYCEROL
| Macromolecule | Name: DIACYL GLYCEROL / type: ligand / ID: 5 / Number of copies: 2 / Formula: DGA |
|---|---|
| Molecular weight | Theoretical: 625.018 Da |
| Chemical component information |  ChemComp-DGA: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 9 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Component - Concentration: 25.0 mM / Component - Name: Hepes / Details: 20 mM Hopes-Na, pH7.5, 0.03% DDM |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: After incubation on the grids at 277K under 100% humidity for 10 s, the grids were bloted for 3.0 s and then plunged frozen into liquid ethane cooled by liquid nitrogen using a Vitrobot.. |
| Details | The DltB protein was purified in DDM, the tetramer fractions from gel filtration column were concentrated to about 10 mg/ml. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.1 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: Details: Crystal structure of a membrane-bound O-acyltransferase. |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.23 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 294021 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: PROJECTION MATCHING |
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-8jem: |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)