[English] 日本語
 Yorodumi
Yorodumi- EMDB-36021: Cryo-EM structure of the Arabidopsis thaliana photosystem I(PSI-L... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Arabidopsis thaliana photosystem I(PSI-LHCII-ST2) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | photosystem I / PSI / CELL CYCLE / PHOTOSYNTHESIS | |||||||||
| Function / homology |  Function and homology information Function and homology informationphotosynthesis, light harvesting in photosystem II / PSII associated light-harvesting complex II / response to desiccation / response to red light / cellular response to abscisic acid stimulus / photosynthetic NADP+ reduction / photosystem I stabilization / cellular response to water deprivation / chloroplast photosystem I / chloroplast stromal thylakoid ...photosynthesis, light harvesting in photosystem II / PSII associated light-harvesting complex II / response to desiccation / response to red light / cellular response to abscisic acid stimulus / photosynthetic NADP+ reduction / photosystem I stabilization / cellular response to water deprivation / chloroplast photosystem I / chloroplast stromal thylakoid / response to low light intensity stimulus / plastoglobule / regulation of stomatal closure / chloroplast membrane / response to far red light / response to high light intensity / chloroplast thylakoid / photosynthesis, light harvesting in photosystem I / thylakoid / apoplast / response to fructose / chloroplast envelope / photosystem I reaction center / photosystem I / photosynthetic electron transport in photosystem I / photosynthetic electron transport chain / photosystem I / photosystem II / plastid / chlorophyll binding / chloroplast thylakoid membrane / positive regulation of reactive oxygen species biosynthetic process / response to light stimulus / photosynthesis / response to cold / chloroplast / 4 iron, 4 sulfur cluster binding / electron transfer activity / oxidoreductase activity / protein stabilization / protein domain specific binding / mRNA binding / magnesium ion binding / mitochondrion / extracellular region / metal ion binding / nucleus / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.79 Å | |||||||||
 Authors Authors | Chen SJB / Wu JH / Sui SF / Zhang LX | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Plant / Year: 2023 Journal: Mol Plant / Year: 2023Title: Regulatory dynamics of the higher-plant PSI-LHCI supercomplex during state transitions. Authors: Jianghao Wu / Shuaijiabin Chen / Chao Wang / Weijun Lin / Chao Huang / Chengxu Fan / Dexian Han / Dandan Lu / Xiumei Xu / SenFang Sui / Lixin Zhang /  Abstract: State transition is a fundamental light acclimation mechanism of photosynthetic organisms in response to the environmental light conditions. This process rebalances the excitation energy between ...State transition is a fundamental light acclimation mechanism of photosynthetic organisms in response to the environmental light conditions. This process rebalances the excitation energy between photosystem I (PSI) and photosystem II through regulated reversible binding of the light-harvesting complex II (LHCII) to PSI. However, the structural reorganization of PSI-LHCI, the dynamic binding of LHCII, and the regulatory mechanisms underlying state transitions are less understood in higher plants. In this study, using cryoelectron microscopy we resolved the structures of PSI-LHCI in both state 1 (PSI-LHCI-ST1) and state 2 (PSI-LHCI-LHCII-ST2) from Arabidopsis thaliana. Combined genetic and functional analyses revealed novel contacts between Lhcb1 and PsaK that further enhanced the binding of the LHCII trimer to the PSI core with the known interactions between phosphorylated Lhcb2 and the PsaL/PsaH/PsaO subunits. Specifically, PsaO was absent in the PSI-LHCI-ST1 supercomplex but present in the PSI-LHCI-LHCII-ST2 supercomplex, in which the PsaL/PsaK/PsaA subunits undergo several conformational changes to strengthen the binding of PsaO in ST2. Furthermore, the PSI-LHCI module adopts a more compact configuration with shorter Mg-to-Mg distances between the chlorophylls, which may enhance the energy transfer efficiency from the peripheral antenna to the PSI core in ST2. Collectively, our work provides novel structural and functional insights into the mechanisms of light acclimation during state transitions in higher plants. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36021.map.gz emd_36021.map.gz | 129.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36021-v30.xml emd-36021-v30.xml emd-36021.xml emd-36021.xml | 38 KB 38 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36021.png emd_36021.png | 15.5 KB | ||
| Filedesc metadata |  emd-36021.cif.gz emd-36021.cif.gz | 10 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36021 http://ftp.pdbj.org/pub/emdb/structures/EMD-36021 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36021 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36021 | HTTPS FTP |
-Related structure data
| Related structure data |  8j6zMC  8j7aC  8j7bC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36021.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36021.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : photosystem I
+Supramolecule #1: photosystem I
+Macromolecule #1: Chlorophyll a-b binding protein 6, chloroplastic
+Macromolecule #2: Photosystem I chlorophyll a/b-binding protein 2, chloroplastic
+Macromolecule #3: Photosystem I chlorophyll a/b-binding protein 3-1, chloroplastic
+Macromolecule #4: Chlorophyll a-b binding protein 4, chloroplastic
+Macromolecule #5: Photosystem I P700 chlorophyll a apoprotein A1
+Macromolecule #6: Photosystem I P700 chlorophyll a apoprotein A2
+Macromolecule #7: Photosystem I iron-sulfur center
+Macromolecule #8: Photosystem I reaction center subunit II-2, chloroplastic
+Macromolecule #9: Photosystem I reaction center subunit IV A, chloroplastic
+Macromolecule #10: Photosystem I reaction center subunit III, chloroplastic
+Macromolecule #11: Photosystem I reaction center subunit V, chloroplastic
+Macromolecule #12: Photosystem I reaction center subunit VI-2, chloroplastic
+Macromolecule #13: Photosystem I reaction center subunit VIII
+Macromolecule #14: Photosystem I reaction center subunit IX
+Macromolecule #15: Photosystem I reaction center subunit psaK, chloroplastic
+Macromolecule #16: Photosystem I reaction center subunit XI, chloroplastic
+Macromolecule #17: Photosystem I subunit O
+Macromolecule #18: Chlorophyll a-b binding protein 2, chloroplastic
+Macromolecule #19: Chlorophyll a-b binding protein 2.1, chloroplastic
+Macromolecule #20: CHLOROPHYLL B
+Macromolecule #21: CHLOROPHYLL A
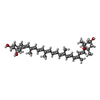
+Macromolecule #22: (3S,5R,6S,3'S,5'R,6'S)-5,6,5',6'-DIEPOXY-5,6,5',6'- TETRAHYDRO-BE...
+Macromolecule #23: 1,2-DIPALMITOYL-PHOSPHATIDYL-GLYCEROLE
+Macromolecule #24: (3R,3'R,6S)-4,5-DIDEHYDRO-5,6-DIHYDRO-BETA,BETA-CAROTENE-3,3'-DIOL
+Macromolecule #25: BETA-CAROTENE
+Macromolecule #26: 1,2-DISTEAROYL-MONOGALACTOSYL-DIGLYCERIDE
+Macromolecule #27: CHLOROPHYLL A ISOMER
+Macromolecule #28: IRON/SULFUR CLUSTER
+Macromolecule #29: PHYLLOQUINONE
+Macromolecule #30: DIGALACTOSYL DIACYL GLYCEROL (DGDG)
+Macromolecule #31: (1R,3R)-6-{(3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1S,4R,6R)-4-HYDROXY...
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.79 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 188490 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)