[English] 日本語
 Yorodumi
Yorodumi- EMDB-35978: Structure of HerA-Sir2 complex from Escherichia coli Nezha system -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of HerA-Sir2 complex from Escherichia coli Nezha system | |||||||||
 Map data Map data | mrc-map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | defense system HerA Sir2 / IMMUNE SYSTEM | |||||||||
| Function / homology | Helicase HerA, central domain / Helicase HerA, central domain / SIR2-like domain / SIR2-like domain / hydrolase activity / P-loop containing nucleoside triphosphate hydrolase / SIR2-like domain-containing protein / Nucleoside triphosphate hydrolase Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
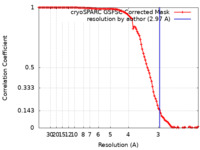
| Method | single particle reconstruction / cryo EM / Resolution: 2.97 Å | |||||||||
 Authors Authors | Chen Q / Yu Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: Multiple enzymatic activities of a Sir2-HerA system cooperate for anti-phage defense. Authors: Dongmei Tang / Yijun Chen / Hao Chen / Tingting Jia / Qiang Chen / Yamei Yu /  Abstract: In response to the persistent exposure to phage infection, bacteria have evolved diverse antiviral defense mechanisms. In this study, we report a bacterial two-component defense system consisting of ...In response to the persistent exposure to phage infection, bacteria have evolved diverse antiviral defense mechanisms. In this study, we report a bacterial two-component defense system consisting of a Sir2 NADase and a HerA helicase. Cryo-electron microscopy reveals that Sir2 and HerA assemble into a ∼1 MDa supramolecular octadecamer. Unexpectedly, this complex exhibits various enzymatic activities, including ATPase, NADase, helicase, and nuclease, which work together in a sophisticated manner to fulfill the antiphage function. Therefore, we name this defense system "Nezha" after a divine warrior in Chinese mythology who employs multiple weapons to defeat enemies. Our findings demonstrate that Nezha could sense phage infections, self-activate to arrest cell growth, eliminate phage genomes, and subsequently deactivate to allow for cell recovery. Collectively, Nezha represents a paradigm of sophisticated and multifaceted strategies bacteria use to defend against viral infections. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35978.map.gz emd_35978.map.gz | 118.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35978-v30.xml emd-35978-v30.xml emd-35978.xml emd-35978.xml | 19.6 KB 19.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35978_fsc.xml emd_35978_fsc.xml | 10.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_35978.png emd_35978.png | 193 KB | ||
| Filedesc metadata |  emd-35978.cif.gz emd-35978.cif.gz | 6.6 KB | ||
| Others |  emd_35978_half_map_1.map.gz emd_35978_half_map_1.map.gz emd_35978_half_map_2.map.gz emd_35978_half_map_2.map.gz | 116.2 MB 116.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35978 http://ftp.pdbj.org/pub/emdb/structures/EMD-35978 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35978 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35978 | HTTPS FTP |
-Related structure data
| Related structure data |  8j4uMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_35978.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35978.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | mrc-map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.11 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: mrc-map
| File | emd_35978_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | mrc-map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half-mapA
| File | emd_35978_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-mapA | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : HerA-Sir2
| Entire | Name: HerA-Sir2 |
|---|---|
| Components |
|
-Supramolecule #1: HerA-Sir2
| Supramolecule | Name: HerA-Sir2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: SIR2-like domain-containing protein
| Macromolecule | Name: SIR2-like domain-containing protein / type: protein_or_peptide / ID: 1 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 46.817664 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSIYQGGNKL NEDDFRSHVY SLCQLDNVGV LLGAGASVGC GGKTMKDVWK SFKQNYPELL GALIDKYLLV SQIDSDNNLV NVELLIDEA TKFLSVAKTR RCEDEEEEFR KILSSLYKEV TKAALLTGEQ FREKNQGKKD AFKYHKELIS KLISNRQPGQ S APAIFTTN ...String: MSIYQGGNKL NEDDFRSHVY SLCQLDNVGV LLGAGASVGC GGKTMKDVWK SFKQNYPELL GALIDKYLLV SQIDSDNNLV NVELLIDEA TKFLSVAKTR RCEDEEEEFR KILSSLYKEV TKAALLTGEQ FREKNQGKKD AFKYHKELIS KLISNRQPGQ S APAIFTTN YDLALEWAAE DLGIQLFNGF SGLHTRQFYP QNFDLAFRNV NAKGEARFGH YHAYLYKLHG SLTWYQNDSL TV NEVSASQ AYDEYINDII NKDDFYRGQH LIYPGANKYS HTIGFVYGEM FRRFGEFISK PQTALFINGF GFGDYHINRI ILG ALLNPS FHVVIYYPEL KEAITKVSKG GGSEAEKAIV TLKNMAFNQV TVVGGGSKAY FNSFVEHLPY PVLFPRDNIV DELV EAIAN LSKGEGNVPF UniProtKB: SIR2-like domain-containing protein |
-Macromolecule #2: Nucleoside triphosphate hydrolase
| Macromolecule | Name: Nucleoside triphosphate hydrolase / type: protein_or_peptide / ID: 2 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 68.431992 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSLFKLTEIS AIGYVVGLEG ERIRINLHEG LQGRLASHRK GVSSVTQPGD LIGFDAGNIL VVARVTDMAF VEADKAHKAN VGTSDLADI PLRQIIAYAI GFVKRELNGY VFISEDWRLP ALGSSAVPLT SDFLNIIYSI DKEELPKAVE LGVDSRTKTV K IFASVDKL ...String: MSLFKLTEIS AIGYVVGLEG ERIRINLHEG LQGRLASHRK GVSSVTQPGD LIGFDAGNIL VVARVTDMAF VEADKAHKAN VGTSDLADI PLRQIIAYAI GFVKRELNGY VFISEDWRLP ALGSSAVPLT SDFLNIIYSI DKEELPKAVE LGVDSRTKTV K IFASVDKL LSRHLAVLGS TGYGKSNFNA LLTRKVSEKY PNSRIVIFDI NGEYAQAFTG IPNVKHTILG ESPNVDSLEK KQ QKGELYS EEYYCYKKIP YQALGFAGLI KLLRPSDKTQ LPALRNALSA INRTHFKSRN IYLEKDDGET FLLYDDCRDT NQS KLAEWL DLLRRRRLKR TNVWPPFKSL ATLVAEFGCV AADRSNGSKR DAFGFSNVLP LVKIIQQLAE DIRFKSIVNL NGGG ELADG GTHWDKAMSD EVDYFFGKEK GQENDWNVHI VNMKNLAQDH APMLLSALLE MFAEILFRRG QERSYPTVLL LEEAH HYLR DPYAEIDSQI KAYERLAKEG RKFKCSLIVS TQRPSELSPT VLAMCSNWFS LRLTNERDLQ ALRYAMESGN EQILKQ ISG LPRGDAVAFG SAFNLPVRIS INQARPGPKS SDAVFSEEWA NCTELRC UniProtKB: Nucleoside triphosphate hydrolase |
-Macromolecule #3: [(2R,3S,4R,5R)-5-(6-AMINOPURIN-9-YL)-3,4-DIHYDROXY-OXOLAN-2-YL]ME...
| Macromolecule | Name: [(2R,3S,4R,5R)-5-(6-AMINOPURIN-9-YL)-3,4-DIHYDROXY-OXOLAN-2-YL]METHYL[HYDROXY-[[(2R,3S,4R,5S)-3,4,5-TRIHYDROXYOXOLAN-2-YL]METHOXY]PHOSPHORYL] HYDROGEN PHOSPHATE type: ligand / ID: 3 / Number of copies: 12 / Formula: AR6 |
|---|---|
| Molecular weight | Theoretical: 559.316 Da |
-Macromolecule #4: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER / type: ligand / ID: 4 / Number of copies: 5 / Formula: AGS |
|---|---|
| Molecular weight | Theoretical: 523.247 Da |
| Chemical component information |  ChemComp-AGS: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)