[English] 日本語
 Yorodumi
Yorodumi- EMDB-35643: Cryo-EM structure of heme transporter CydDC from Escherichia coli... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of heme transporter CydDC from Escherichia coli in the occluded ATP bound state | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | ABC transporter / Heme / TRANSPORT PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcysteine transport / glutathione transmembrane transport / ABC-type oligopeptide transporter activity / ATPase-coupled lipid transmembrane transporter activity / ABC-type transporter activity / cell redox homeostasis / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||||||||
 Authors Authors | Zhu C / Li J | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Protein Cell / Year: 2023 Journal: Protein Cell / Year: 2023Title: Cryo-EM structures of a prokaryotic heme transporter CydDC. Authors: Chen Zhu / Yanfeng Shi / Jing Yu / Wenhao Zhao / Lingqiao Li / Jingxi Liang / Xiaolin Yang / Bing Zhang / Yao Zhao / Yan Gao / Xiaobo Chen / Xiuna Yang / Lu Zhang / Luke W Guddat / Lei Liu / ...Authors: Chen Zhu / Yanfeng Shi / Jing Yu / Wenhao Zhao / Lingqiao Li / Jingxi Liang / Xiaolin Yang / Bing Zhang / Yao Zhao / Yan Gao / Xiaobo Chen / Xiuna Yang / Lu Zhang / Luke W Guddat / Lei Liu / Haitao Yang / Zihe Rao / Jun Li /   | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35643.map.gz emd_35643.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35643-v30.xml emd-35643-v30.xml emd-35643.xml emd-35643.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35643.png emd_35643.png | 44.1 KB | ||
| Filedesc metadata |  emd-35643.cif.gz emd-35643.cif.gz | 6.1 KB | ||
| Others |  emd_35643_half_map_1.map.gz emd_35643_half_map_1.map.gz emd_35643_half_map_2.map.gz emd_35643_half_map_2.map.gz | 116.1 MB 116.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35643 http://ftp.pdbj.org/pub/emdb/structures/EMD-35643 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35643 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35643 | HTTPS FTP |
-Related structure data
| Related structure data |  8iptMC  8ipqC  8iprC  8ipsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35643.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35643.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.96 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_35643_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
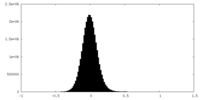
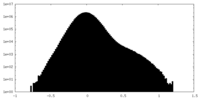
| Density Histograms |
-Half map: #1
| File | emd_35643_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
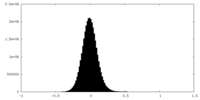
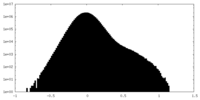
| Density Histograms |
- Sample components
Sample components
-Entire : CydDC
| Entire | Name: CydDC |
|---|---|
| Components |
|
-Supramolecule #1: CydDC
| Supramolecule | Name: CydDC / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: ABC transporter, CydDC cysteine exporter (CydDC-E) family, permea...
| Macromolecule | Name: ABC transporter, CydDC cysteine exporter (CydDC-E) family, permease/ATP-binding protein CydC type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 62.978914 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRALLPYLAL YKRHKWMLSL GIVLAIVTLL ASIGLLTLSG WFLSASAVAG VAGLYSFNYM LPAAGVRGAA ITRTAGRYFE RLVSHDATF RVLQHLRIYT FSKLLPLSPA GLARYRQGEL LNRVVADVDT LDHLYLRVIS PLVGAFVVIM VVTIGLSFLD F TLAFTLGG ...String: MRALLPYLAL YKRHKWMLSL GIVLAIVTLL ASIGLLTLSG WFLSASAVAG VAGLYSFNYM LPAAGVRGAA ITRTAGRYFE RLVSHDATF RVLQHLRIYT FSKLLPLSPA GLARYRQGEL LNRVVADVDT LDHLYLRVIS PLVGAFVVIM VVTIGLSFLD F TLAFTLGG IMLLTLFLMP PLFYRAGKST GQNLTHLRGQ YRQQLTAWLQ GQAELTIFGA SDRYRTQLEN TEIQWLEAQR RQ SELTALS QAIMLLIGAL AVILMLWMAS GGVGGNAQPG ALIALFVFCA LAAFEALAPV TGAFQHLGQV IASAVRITDL TDQ KPEVTF PDTQTRVADR VSLTLRDVQF TYPEQSQQAL KGISLQVNAG EHIAILGRTG CGKSTLLQLL TRAWDPQQGE ILLN DSPIA SLNEAALRQT ISVVPQRVHL FSATLRDNLL LASPGSSDEA LSEILRRVGL EKLLEDAGLN SWLGEGGRQL SGGEL RRLA IARALLHDAP LVLLDQPTEG LDATTESQIL ELLAEMMREK TVLMVTHRLR GLSRFQQIIV MDNGQIIEQG THAELL ARQ GRYYQFKQGL UniProtKB: ABC transporter, CydDC cysteine exporter (CydDC-E) family, permease/ATP-binding protein CydC |
-Macromolecule #2: ABC transporter, CydDC cysteine exporter (CydDC-E) family, permea...
| Macromolecule | Name: ABC transporter, CydDC cysteine exporter (CydDC-E) family, permease/ATP-binding protein CydD type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.143953 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNKSRQKELT RWLKQQSVIS QRWLNISRLL GFVSGILIIA QAWFMARILQ HMIMENIPRE ALLLPFTLLV LTFVLRAWVV WLRERVGYH AGQHIRFAIR RQVLDRLQQA GPAWIQGKPA GSWATLVLEQ IDDMHDYYAR YLPQMALAVS VPLLIVVAIF P SNWAAALI ...String: MNKSRQKELT RWLKQQSVIS QRWLNISRLL GFVSGILIIA QAWFMARILQ HMIMENIPRE ALLLPFTLLV LTFVLRAWVV WLRERVGYH AGQHIRFAIR RQVLDRLQQA GPAWIQGKPA GSWATLVLEQ IDDMHDYYAR YLPQMALAVS VPLLIVVAIF P SNWAAALI LLGTAPLIPL FMALVGMGAA DANRRNFLAL ARLSGHFLDR LRGMETLRIF GRGEAEIESI RSASEDFRQR TM EVLRLAF LSSGILEFFT SLSIALVAVY FGFSYLGELD FGHYDTGVTL AAGFLALILA PEFFQPLRDL GTFYHAKAQA VGA ADSLKT FMETPLAHPQ RGEAELALTD PLTIEAEDLF ITSPEGKTLA GPLNFTLPAG QRAVLVGRSG SGKSSLLNAL SGFL SYQGS LRINGIELRD LSPESWRKHL SWVGQNPQLP AATLRDNVLL ARPDASEQEL QAALDNAWVS EFLPLLPQGV DTPVG DQAA RLSVGQAQRV AVARALLNPC SLLLLDQPAA SLDAHSEQRV MEALNAASLR QTTLMVTHQL EDLADWDVIW VMQDGR IIE QGRYAELSVA GGPFATLLAH RQEEI UniProtKB: ABC transporter, CydDC cysteine exporter (CydDC-E) family, permease/ATP-binding protein CydD |
-Macromolecule #3: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 4 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 119296 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)