[English] 日本語
 Yorodumi
Yorodumi- EMDB-35420: Cryo-EM structure of tetrameric SPARTA gRNA-ssDNA target complex ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of tetrameric SPARTA gRNA-ssDNA target complex in state1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA BINDING PROTEIN-RNA-DNA COMPLEX | |||||||||
| Function / homology | TIR domain / Toll/interleukin-1 receptor homology (TIR) domain / Toll/interleukin-1 receptor homology (TIR) domain superfamily / Ribonuclease H superfamily / nucleic acid binding / Ribonuclease H-like superfamily / signal transduction / Piwi domain-containing protein / TIR domain-containing protein Function and homology information Function and homology information | |||||||||
| Biological species |  Thermoflavifilum thermophilum (bacteria) / Thermoflavifilum thermophilum (bacteria) /  | |||||||||
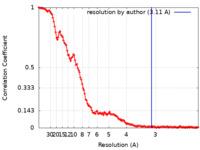
| Method | single particle reconstruction / cryo EM / Resolution: 3.11 Å | |||||||||
 Authors Authors | Zhang JT / Jia N / Huang HD | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2024 Journal: Nat Chem Biol / Year: 2024Title: Target ssDNA activates the NADase activity of prokaryotic SPARTA immune system. Authors: Jun-Tao Zhang / Xin-Yang Wei / Ning Cui / Ruilin Tian / Ning Jia /  Abstract: Argonaute proteins (Agos), which use small RNAs or DNAs as guides to recognize complementary nucleic acid targets, mediate RNA silencing in eukaryotes. In prokaryotes, Agos are involved in immunity: ...Argonaute proteins (Agos), which use small RNAs or DNAs as guides to recognize complementary nucleic acid targets, mediate RNA silencing in eukaryotes. In prokaryotes, Agos are involved in immunity: the short prokaryotic Ago/TIR-APAZ (SPARTA) immune system triggers cell death by degrading NAD in response to invading plasmids, but its molecular mechanisms remain unknown. Here we used cryo-electron microscopy to determine the structures of inactive monomeric and active tetrameric Crenotalea thermophila SPARTA complexes, revealing mechanisms underlying SPARTA assembly, RNA-guided recognition of target single-stranded DNA (ssDNA) and subsequent SPARTA tetramerization, as well as tetramerization-dependent NADase activation. The small RNA guides Ago to recognize its ssDNA target, inducing SPARTA tetramerization via both Ago- and TIR-mediated interactions and resulting in a two-stranded, parallel, head-to-tail TIR rearrangement primed for NAD hydrolysis. Our findings thus identify the molecular basis for target ssDNA-mediated SPARTA activation, which will facilitate the development of SPARTA-based biotechnological tools. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35420.map.gz emd_35420.map.gz | 168.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35420-v30.xml emd-35420-v30.xml emd-35420.xml emd-35420.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35420_fsc.xml emd_35420_fsc.xml | 16.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_35420.png emd_35420.png | 46.4 KB | ||
| Masks |  emd_35420_msk_1.map emd_35420_msk_1.map | 178 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-35420.cif.gz emd-35420.cif.gz | 6.3 KB | ||
| Others |  emd_35420_half_map_1.map.gz emd_35420_half_map_1.map.gz emd_35420_half_map_2.map.gz emd_35420_half_map_2.map.gz | 165.1 MB 165.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35420 http://ftp.pdbj.org/pub/emdb/structures/EMD-35420 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35420 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35420 | HTTPS FTP |
-Validation report
| Summary document |  emd_35420_validation.pdf.gz emd_35420_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35420_full_validation.pdf.gz emd_35420_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  emd_35420_validation.xml.gz emd_35420_validation.xml.gz | 20 KB | Display | |
| Data in CIF |  emd_35420_validation.cif.gz emd_35420_validation.cif.gz | 26.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35420 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35420 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35420 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35420 | HTTPS FTP |
-Related structure data
| Related structure data |  8iflMC  8ifkC  8ifmC  8k34C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35420.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35420.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
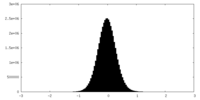
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_35420_msk_1.map emd_35420_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
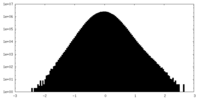
| Density Histograms |
-Half map: #2
| File | emd_35420_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_35420_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CrtSPARTA tetramer complex
| Entire | Name: CrtSPARTA tetramer complex |
|---|---|
| Components |
|
-Supramolecule #1: CrtSPARTA tetramer complex
| Supramolecule | Name: CrtSPARTA tetramer complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Thermoflavifilum thermophilum (bacteria) Thermoflavifilum thermophilum (bacteria) |
-Macromolecule #1: TIR domain-containing protein
| Macromolecule | Name: TIR domain-containing protein / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Thermoflavifilum thermophilum (bacteria) Thermoflavifilum thermophilum (bacteria) |
| Molecular weight | Theoretical: 53.256734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRNKIFISHA TPEDDDFTRW LSLKLIGLGY EVWCDILFLD KGVDFWSTIE KEIRENTCKF LIVSSTAGNK REGVLKELAV ATKVKKHLQ DDMFIIPLAI DENLSYDDIN IEIVRLNAID FKKSWAKGLQ DLLDAFEKQN VPKKPPDHSK SNLLYQQIFL H DKQAIEKE ...String: MRNKIFISHA TPEDDDFTRW LSLKLIGLGY EVWCDILFLD KGVDFWSTIE KEIRENTCKF LIVSSTAGNK REGVLKELAV ATKVKKHLQ DDMFIIPLAI DENLSYDDIN IEIVRLNAID FKKSWAKGLQ DLLDAFEKQN VPKKPPDHSK SNLLYQQIFL H DKQAIEKE ETYDSNWFPI ISFPNELRFH RYDWRLPKQF DVRTLAFPAI RYKEYLCTFA WEYDFIHQLP KTETYNGQES IR ISTSDIL SGRYDTDFIR NYECQRLIVQ LINKAFELRM KDKNVREYQM SKTFAYWIEK GKLEKDKFEK IKLVGKQKNK YWH FGISAA GKLYPSPVLM VSSHIIFTMD GINLIKSKSI QHSSRRKQGK NWWNDKWREK LLAFIRFLSD DQNAIYLNVG SEEK ILISN KPLKFFGKMS YVTPSEVTLE EESVLADINN FEEDTEDLDE LEDIE UniProtKB: TIR domain-containing protein |
-Macromolecule #2: Piwi domain-containing protein
| Macromolecule | Name: Piwi domain-containing protein / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Thermoflavifilum thermophilum (bacteria) Thermoflavifilum thermophilum (bacteria) |
| Molecular weight | Theoretical: 58.304848 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKELIYIEEP SILFAHGQKC TDPRDGLALF GPLNQIYGIK SGVVGTQKGL QIFKSYLDKI QKPIYNHNNI TRPMFPGFEA VFGCKWESQ NIVFKEITDE EIRRYLFNAS THKRTYDLVT LFNDKIITAN KNDEERVDVW FVIVPEEIYK YCRPNSVLPN E LVQTKSLI ...String: MKELIYIEEP SILFAHGQKC TDPRDGLALF GPLNQIYGIK SGVVGTQKGL QIFKSYLDKI QKPIYNHNNI TRPMFPGFEA VFGCKWESQ NIVFKEITDE EIRRYLFNAS THKRTYDLVT LFNDKIITAN KNDEERVDVW FVIVPEEIYK YCRPNSVLPN E LVQTKSLI SKSKAKSFRY TPTLFEEFNK KLKEVEKEAK TYNYDAQFHD QLKARLLEHT IPTQILREST LAWRDFKNTF GA PIRDFSK IEGHLAWTIS TAAYYKAGGK PWKLGDIRPG VCYLGLVYKK IEKSKNPQNA CCAAQMFLDN GDGTVFKGEV GPW YNPEKG EYHLKPKEAK ALLTQALESY KEQNKSYPKE VFIHARTRFN DEEWNAFNEV TPKNTNLVGV TITKSKPLKL YKTE GAFPI MRGNAYIVDE KKAFLWTLGF VPKLQSTLSM EVPNPIFIEI NKGEAEIQQV LKDILALTKL NYNACIYADG EPVTL RFAN KIGEILTAST EIKTPPLAFK YYI UniProtKB: Piwi domain-containing protein |
-Macromolecule #3: guide RNA
| Macromolecule | Name: guide RNA / type: rna / ID: 3 / Number of copies: 4 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 6.658989 KDa |
| Sequence | String: AAACGGCUCU AAUCUAUUAG U |
-Macromolecule #4: target ssDNA
| Macromolecule | Name: target ssDNA / type: dna / ID: 4 / Number of copies: 4 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.680997 KDa |
| Sequence | String: (DC)(DA)(DA)(DC)(DT)(DA)(DA)(DT)(DA)(DG) (DA)(DT)(DT)(DA)(DG)(DA)(DG)(DC)(DC)(DG) (DT)(DT)(DT)(DA)(DT) |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 4 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)