+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Clr6 HDAC (Clr6S)-nucleosome complex | |||||||||
 Map data Map data | map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NCP / PTM / modification / TRANSCRIPTION | |||||||||
| Biological species |  | |||||||||
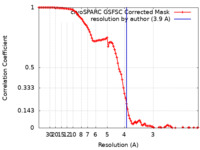
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Zhang HQ | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Class I histone deacetylase complex: Structure and functional correlates. Authors: Xiao Wang / Yannan Wang / Simiao Liu / Yi Zhang / Ke Xu / Liting Ji / Roger D Kornberg / Heqiao Zhang /   Abstract: The Clr6S complex, a class I histone deacetylase complex, functions as a zinc-dependent enzyme to remove acetyl groups from lysine residues in histone tails. We report here the cryo-EM structure of ...The Clr6S complex, a class I histone deacetylase complex, functions as a zinc-dependent enzyme to remove acetyl groups from lysine residues in histone tails. We report here the cryo-EM structure of Clr6S alone and a cryo-EM map of Clr6S in complex with a nucleosome. The active center, revealed at near-atomic resolution, includes features important for catalysis-A water molecule coordinated by zinc, the likely nucleophile for attack on the acetyl-lysine bond, and a loop that may position the substrate for catalysis. The cryo-EM map in the presence of a nucleosome reveals multiple Clr6S-nucleosome contacts and a high degree of relative motion of Clr6S and the nucleosome. Such flexibility may be attributed to interaction at a site in the flexible histone tail and is likely important for the function of the deacetylase, which acts at multiple sites in other histone tails. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35417.map.gz emd_35417.map.gz | 51.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35417-v30.xml emd-35417-v30.xml emd-35417.xml emd-35417.xml | 11.5 KB 11.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35417_fsc.xml emd_35417_fsc.xml | 11.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_35417.png emd_35417.png | 43.4 KB | ||
| Filedesc metadata |  emd-35417.cif.gz emd-35417.cif.gz | 3.6 KB | ||
| Others |  emd_35417_half_map_1.map.gz emd_35417_half_map_1.map.gz emd_35417_half_map_2.map.gz emd_35417_half_map_2.map.gz | 95.5 MB 95.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35417 http://ftp.pdbj.org/pub/emdb/structures/EMD-35417 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35417 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35417 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_35417.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35417.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half map
| File | emd_35417_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
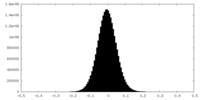
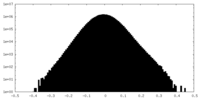
| Density Histograms |
-Half map: half map
| File | emd_35417_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
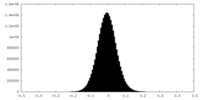
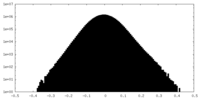
| Density Histograms |
- Sample components
Sample components
-Entire : Chromatin-related complex-NCP
| Entire | Name: Chromatin-related complex-NCP |
|---|---|
| Components |
|
-Supramolecule #1: Chromatin-related complex-NCP
| Supramolecule | Name: Chromatin-related complex-NCP / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)