+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bre1(FL)-Rad6-nucleosome complex | |||||||||
 Map data Map data | E3-E2-NCP | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Nucleosome / Bre1 / Rad6 / NUCLEAR PROTEIN | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.36 Å | |||||||||
 Authors Authors | Ai H / Deng Z / Pan M / Liu L | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: Mechanistic insights into nucleosomal H2B monoubiquitylation mediated by yeast Bre1-Rad6 and its human homolog RNF20/RNF40-hRAD6A. Authors: Zhiheng Deng / Huasong Ai / Maoshen Sun / Zebin Tong / Yunxiang Du / Qian Qu / Liying Zhang / Ziyu Xu / Shixian Tao / Qiang Shi / Jia-Bin Li / Man Pan / Lei Liu /  Abstract: Histone H2B monoubiquitylation plays essential roles in chromatin-based transcriptional processes. A RING-type E3 ligase (yeast Bre1 or human RNF20/RNF40) and an E2 ubiquitin-conjugating enzyme ...Histone H2B monoubiquitylation plays essential roles in chromatin-based transcriptional processes. A RING-type E3 ligase (yeast Bre1 or human RNF20/RNF40) and an E2 ubiquitin-conjugating enzyme (yeast Rad6 or human hRAD6A), together, precisely deposit ubiquitin on H2B K123 in yeast or K120 in humans. Here, we developed a chemical trapping strategy and successfully captured the transient structures of Bre1- or RNF20/RNF40-mediated ubiquitin transfer from Rad6 or hRAD6A to nucleosomal H2B. Our structures show that Bre1 and RNF40 directly bind nucleosomal DNA, exhibiting a conserved E3/E2/nucleosome interaction pattern from yeast to humans for H2B monoubiquitylation. We also find an uncanonical non-hydrophobic contact in the Bre1 RING-Rad6 interface, which positions Rad6 directly above the target H2B lysine residue. Our study provides mechanistic insights into the site-specific monoubiquitylation of H2B, reveals a critical role of nucleosomal DNA in mediating E3 ligase recognition, and provides a framework for understanding the cancer-driving mutations of RNF20/RNF40. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35379.map.gz emd_35379.map.gz | 4.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35379-v30.xml emd-35379-v30.xml emd-35379.xml emd-35379.xml | 14.1 KB 14.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35379.png emd_35379.png | 104.5 KB | ||
| Others |  emd_35379_additional_1.map.gz emd_35379_additional_1.map.gz emd_35379_half_map_1.map.gz emd_35379_half_map_1.map.gz emd_35379_half_map_2.map.gz emd_35379_half_map_2.map.gz | 4.5 MB 49.6 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35379 http://ftp.pdbj.org/pub/emdb/structures/EMD-35379 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35379 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35379 | HTTPS FTP |
-Validation report
| Summary document |  emd_35379_validation.pdf.gz emd_35379_validation.pdf.gz | 757.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35379_full_validation.pdf.gz emd_35379_full_validation.pdf.gz | 756.8 KB | Display | |
| Data in XML |  emd_35379_validation.xml.gz emd_35379_validation.xml.gz | 12.1 KB | Display | |
| Data in CIF |  emd_35379_validation.cif.gz emd_35379_validation.cif.gz | 14.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35379 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35379 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35379 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35379 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_35379.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35379.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E3-E2-NCP | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.074 Å | ||||||||||||||||||||||||||||||||||||
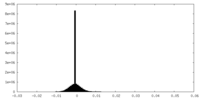
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: E3-NCP
| File | emd_35379_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E3-NCP | ||||||||||||
| Projections & Slices |
| ||||||||||||
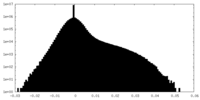
| Density Histograms |
-Half map: #1
| File | emd_35379_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_35379_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of full-length Bre1 and Rad6-Ub bound to the nucleosome
| Entire | Name: Complex of full-length Bre1 and Rad6-Ub bound to the nucleosome |
|---|---|
| Components |
|
-Supramolecule #1: Complex of full-length Bre1 and Rad6-Ub bound to the nucleosome
| Supramolecule | Name: Complex of full-length Bre1 and Rad6-Ub bound to the nucleosome type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.36 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 17255 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)