[English] 日本語
 Yorodumi
Yorodumi- EMDB-35001: E. coli 70S ribosome complexed with tRNA_Ile2 bearing L34 and t6A... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli 70S ribosome complexed with tRNA_Ile2 bearing L34 and t6A37 in classical state | ||||||||||||||||||
 Map data Map data | Before post-processing | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | tRNA modification / decoding / RIBOSOME | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of cytoplasmic translational initiation / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity ...negative regulation of cytoplasmic translational initiation / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / negative regulation of cytoplasmic translation / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / negative regulation of translational initiation / regulation of mRNA stability / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / assembly of large subunit precursor of preribosome / positive regulation of RNA splicing / cytosolic ribosome assembly / regulation of DNA-templated transcription elongation / response to reactive oxygen species / ribosome assembly / transcription elongation factor complex / DNA endonuclease activity / transcription antitermination / regulation of cell growth / translational initiation / DNA-templated transcription termination / response to radiation / maintenance of translational fidelity / mRNA 5'-UTR binding / regulation of translation / large ribosomal subunit / ribosome biogenesis / ribosome binding / transferase activity / ribosomal small subunit biogenesis / ribosomal small subunit assembly / small ribosomal subunit / 5S rRNA binding / ribosomal large subunit assembly / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / membrane / cytoplasm / cytosol Similarity search - Function | ||||||||||||||||||
| Biological species |   | ||||||||||||||||||
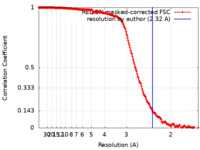
| Method | single particle reconstruction / cryo EM / Resolution: 2.32 Å | ||||||||||||||||||
 Authors Authors | Akiyama N / Ishiguro K / Yokoyama T / Shirouzu M / Suzuki T | ||||||||||||||||||
| Funding support |  Japan, 5 items Japan, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structural insights into the decoding capability of isoleucine tRNAs with lysidine and agmatidine. Authors: Naho Akiyama / Kensuke Ishiguro / Takeshi Yokoyama / Kenjyo Miyauchi / Asuteka Nagao / Mikako Shirouzu / Tsutomu Suzuki /  Abstract: The anticodon modifications of transfer RNAs (tRNAs) finetune the codon recognition on the ribosome for accurate translation. Bacteria and archaea utilize the modified cytidines, lysidine (L) and ...The anticodon modifications of transfer RNAs (tRNAs) finetune the codon recognition on the ribosome for accurate translation. Bacteria and archaea utilize the modified cytidines, lysidine (L) and agmatidine (agmC), respectively, in the anticodon of tRNA to decipher AUA codon. L and agmC contain long side chains with polar termini, but their functions remain elusive. Here we report the cryogenic electron microscopy structures of tRNAs recognizing the AUA codon on the ribosome. Both modifications interact with the third adenine of the codon via a unique C-A geometry. The side chains extend toward 3' direction of the mRNA, and the polar termini form hydrogen bonds with 2'-OH of the residue 3'-adjacent to the AUA codon. Biochemical analyses demonstrated that AUA decoding is facilitated by the additional interaction between the polar termini of the modified cytidines and 2'-OH of the fourth mRNA residue. We also visualized cyclic N-threonylcarbamoyladenosine (ctA), another tRNA modification, and revealed a molecular basis how ctA contributes to efficient decoding. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35001.map.gz emd_35001.map.gz | 531.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35001-v30.xml emd-35001-v30.xml emd-35001.xml emd-35001.xml | 89.1 KB 89.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35001_fsc.xml emd_35001_fsc.xml | 18.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_35001.png emd_35001.png | 77.5 KB | ||
| Filedesc metadata |  emd-35001.cif.gz emd-35001.cif.gz | 15.7 KB | ||
| Others |  emd_35001_additional_1.map.gz emd_35001_additional_1.map.gz emd_35001_half_map_1.map.gz emd_35001_half_map_1.map.gz emd_35001_half_map_2.map.gz emd_35001_half_map_2.map.gz | 459.1 MB 461 MB 461 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35001 http://ftp.pdbj.org/pub/emdb/structures/EMD-35001 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35001 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35001 | HTTPS FTP |
-Related structure data
| Related structure data |  8hspMC  8htzC  8hu1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35001.map.gz / Format: CCP4 / Size: 567.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35001.map.gz / Format: CCP4 / Size: 567.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Before post-processing | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8285 Å | ||||||||||||||||||||||||||||||||||||
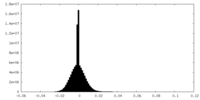
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Before post-processing
| File | emd_35001_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Before post-processing | ||||||||||||
| Projections & Slices |
| ||||||||||||
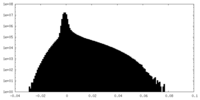
| Density Histograms |
-Half map: #2
| File | emd_35001_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
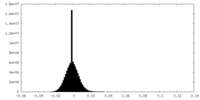
| Density Histograms |
-Half map: #1
| File | emd_35001_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
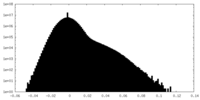
| Density Histograms |
- Sample components
Sample components
+Entire : The complex of E. coli 70S ribosome with mRNA and A-, P- site tRNA
+Supramolecule #1: The complex of E. coli 70S ribosome with mRNA and A-, P- site tRNA
+Supramolecule #2: E. coli 70S ribosome
+Supramolecule #3: A-, P- site tRNA
+Supramolecule #4: mRNA
+Macromolecule #1: 16S rRNA
+Macromolecule #22: 23S rRNA
+Macromolecule #23: 5S rRNA
+Macromolecule #53: P-site tRNA_Glu
+Macromolecule #54: mRNA
+Macromolecule #55: A-site tRNA_Ile2
+Macromolecule #2: 30S ribosomal protein S2
+Macromolecule #3: 30S ribosomal protein S3
+Macromolecule #4: 30S ribosomal protein S4
+Macromolecule #5: 30S ribosomal protein S5
+Macromolecule #6: 30S ribosomal protein S6, fully modified isoform
+Macromolecule #7: 30S ribosomal protein S7
+Macromolecule #8: 30S ribosomal protein S8
+Macromolecule #9: 30S ribosomal protein S9
+Macromolecule #10: 30S ribosomal protein S10
+Macromolecule #11: 30S ribosomal protein S11
+Macromolecule #12: 30S ribosomal protein S12
+Macromolecule #13: 30S ribosomal protein S13
+Macromolecule #14: 30S ribosomal protein S14
+Macromolecule #15: 30S ribosomal protein S15
+Macromolecule #16: 30S ribosomal protein S16
+Macromolecule #17: 30S ribosomal protein S17
+Macromolecule #18: 30S ribosomal protein S18
+Macromolecule #19: 30S ribosomal protein S19
+Macromolecule #20: 30S ribosomal protein S20
+Macromolecule #21: 30S ribosomal protein S21
+Macromolecule #24: 50S ribosomal protein L2
+Macromolecule #25: 50S ribosomal protein L3
+Macromolecule #26: 50S ribosomal protein L4
+Macromolecule #27: 50S ribosomal protein L5
+Macromolecule #28: 50S ribosomal protein L6
+Macromolecule #29: 50S ribosomal protein L9
+Macromolecule #30: 50S ribosomal protein L13
+Macromolecule #31: 50S ribosomal protein L14
+Macromolecule #32: 50S ribosomal protein L15
+Macromolecule #33: 50S ribosomal protein L16
+Macromolecule #34: 50S ribosomal protein L17
+Macromolecule #35: 50S ribosomal protein L18
+Macromolecule #36: 50S ribosomal protein L19
+Macromolecule #37: 50S ribosomal protein L20
+Macromolecule #38: 50S ribosomal protein L21
+Macromolecule #39: 50S ribosomal protein L22
+Macromolecule #40: 50S ribosomal protein L23
+Macromolecule #41: 50S ribosomal protein L24
+Macromolecule #42: 50S ribosomal protein L25
+Macromolecule #43: 50S ribosomal protein L27
+Macromolecule #44: 50S ribosomal protein L28
+Macromolecule #45: 50S ribosomal protein L29
+Macromolecule #46: 50S ribosomal protein L30
+Macromolecule #47: 50S ribosomal protein L32
+Macromolecule #48: 50S ribosomal protein L33
+Macromolecule #49: 50S ribosomal protein L34
+Macromolecule #50: 50S ribosomal protein L35
+Macromolecule #51: 50S ribosomal protein L36
+Macromolecule #52: 50S ribosomal protein L31
+Macromolecule #56: MAGNESIUM ION
+Macromolecule #57: POTASSIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 Component:
Details: The Buffer pH was adjusted to 7.6 using KOH. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 70 sec. | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||
| Details | 100nM ribosomes were incubated with 500nM tRNAs and mRNA |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 6914 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)