+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
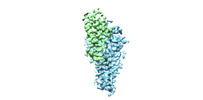
| Title | Cryo-EM structure of human ZDHHC9/GCP16 complex | |||||||||
 Map data Map data | the refined map of human DHHC9/GCP16 complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | plamitoytransferase / palmitoylation / DHHC / RAS / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationRas palmitoyltransferase activity / palmitoyltransferase complex / peptidyl-L-cysteine S-palmitoylation / protein palmitoylation / protein S-acyltransferase / protein-cysteine S-palmitoyltransferase activity / palmitoyltransferase activity / positive regulation of pyroptotic inflammatory response / Golgi to plasma membrane transport / positive regulation of cGAS/STING signaling pathway ...Ras palmitoyltransferase activity / palmitoyltransferase complex / peptidyl-L-cysteine S-palmitoylation / protein palmitoylation / protein S-acyltransferase / protein-cysteine S-palmitoyltransferase activity / palmitoyltransferase activity / positive regulation of pyroptotic inflammatory response / Golgi to plasma membrane transport / positive regulation of cGAS/STING signaling pathway / host-mediated activation of viral process / Golgi to plasma membrane protein transport / Golgi stack / protein targeting to membrane / endoplasmic reticulum-Golgi intermediate compartment membrane / RAS processing / tertiary granule lumen / MAPK cascade / Maturation of spike protein / protein stabilization / Golgi membrane / Neutrophil degranulation / endoplasmic reticulum membrane / endoplasmic reticulum / Golgi apparatus / extracellular exosome / extracellular region / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Wu J / Hu Q / Zhang Y / Liu S / Yang A | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Regulation of RAS palmitoyltransferases by accessory proteins and palmitoylation. Authors: Anlan Yang / Shengjie Liu / Yuqi Zhang / Jia Chen / Yujing Fan / Fengxiang Wang / Yilong Zou / Shan Feng / Jianping Wu / Qi Hu /  Abstract: Palmitoylation of cysteine residues at the C-terminal hypervariable regions in human HRAS and NRAS, which is necessary for RAS signaling, is catalyzed by the acyltransferase DHHC9 in complex with its ...Palmitoylation of cysteine residues at the C-terminal hypervariable regions in human HRAS and NRAS, which is necessary for RAS signaling, is catalyzed by the acyltransferase DHHC9 in complex with its accessory protein GCP16. The molecular basis for the acyltransferase activity and the regulation of DHHC9 by GCP16 is not clear. Here we report the cryo-electron microscopy structures of the human DHHC9-GCP16 complex and its yeast counterpart-the Erf2-Erf4 complex, demonstrating that GCP16 and Erf4 are not directly involved in the catalytic process but stabilize the architecture of DHHC9 and Erf2, respectively. We found that a phospholipid binding to an arginine-rich region of DHHC9 and palmitoylation on three residues (C24, C25 and C288) were essential for the catalytic activity of the DHHC9-GCP16 complex. Moreover, we showed that GCP16 also formed complexes with DHHC14 and DHHC18 to catalyze RAS palmitoylation. These findings provide insights into the regulatory mechanism of RAS palmitoyltransferases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34711.map.gz emd_34711.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34711-v30.xml emd-34711-v30.xml emd-34711.xml emd-34711.xml | 19 KB 19 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34711.png emd_34711.png | 26.2 KB | ||
| Filedesc metadata |  emd-34711.cif.gz emd-34711.cif.gz | 6.3 KB | ||
| Others |  emd_34711_half_map_1.map.gz emd_34711_half_map_1.map.gz emd_34711_half_map_2.map.gz emd_34711_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34711 http://ftp.pdbj.org/pub/emdb/structures/EMD-34711 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34711 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34711 | HTTPS FTP |
-Related structure data
| Related structure data |  8hf3MC  8hfcC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_34711.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34711.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | the refined map of human DHHC9/GCP16 complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8389 Å | ||||||||||||||||||||||||||||||||||||
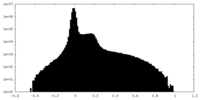
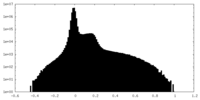
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: the half A map of human DHHC9/GCP16 complex
| File | emd_34711_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | the half A map of human DHHC9/GCP16 complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
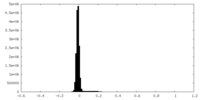
| Density Histograms |
-Half map: the half B map of human DHHC9/GCP16 complex
| File | emd_34711_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | the half B map of human DHHC9/GCP16 complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
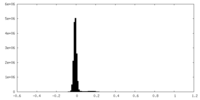
| Density Histograms |
- Sample components
Sample components
-Entire : Human ZDHHC9/GCP16 complex
| Entire | Name: Human ZDHHC9/GCP16 complex |
|---|---|
| Components |
|
-Supramolecule #1: Human ZDHHC9/GCP16 complex
| Supramolecule | Name: Human ZDHHC9/GCP16 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Palmitoyltransferase ZDHHC9
| Macromolecule | Name: Palmitoyltransferase ZDHHC9 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: protein S-acyltransferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.954457 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSVMVVRKKV TRKWEKLPGR NTFCCDGRVM MARQKGIFYL TLFLILGTCT LFFAFECRYL AVQLSPAIPV FAAMLFLFSM ATLLRTSFS DPGVIPRALP DEAAFIEMEI EATNGAVPQG QRPPPRIKNF QINNQIVKLK YCYTCKIFRP PRASHCSICD N CVERFDHH ...String: MSVMVVRKKV TRKWEKLPGR NTFCCDGRVM MARQKGIFYL TLFLILGTCT LFFAFECRYL AVQLSPAIPV FAAMLFLFSM ATLLRTSFS DPGVIPRALP DEAAFIEMEI EATNGAVPQG QRPPPRIKNF QINNQIVKLK YCYTCKIFRP PRASHCSICD N CVERFDHH CPWVGNCVGK RNYRYFYLFI LSLSLLTIYV FAFNIVYVAL KSLKIGFLET LKETPGTVLE VLICFFTLWS VV GLTGFHT FLVALNQTTN EDIKGSWTGK NRVQNPYSHG NIVKNCCEVL CGPLPPSVLD RRGILPLEES GSRPPSTQET SSS LLPQSP APTEHLNSNE MPEDSSTPEE MPPPEPPEPP QEAAEAEKDY KDDDDK UniProtKB: Palmitoyltransferase ZDHHC9 |
-Macromolecule #2: Golgin subfamily A member 7
| Macromolecule | Name: Golgin subfamily A member 7 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 16.803133 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MHHHHHHMRP QQAPVSGKVF IQRDYSSGTR CQFQTKFPAE LENRIDRQQF EETVRTLNNL YAEAEKLGGQ SYLEGCLACL TAYTIFLCM ETHYEKVLKK VSKYIQEQNE KIYAPQGLLL TDPIERGLRV IEITIYEDRG MSSGR UniProtKB: Golgin subfamily A member 7 |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #4: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 4 / Number of copies: 4 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Macromolecule #5: 1,2-DILAUROYL-SN-GLYCERO-3-PHOSPHATE
| Macromolecule | Name: 1,2-DILAUROYL-SN-GLYCERO-3-PHOSPHATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: PX2 |
|---|---|
| Molecular weight | Theoretical: 535.671 Da |
| Chemical component information |  ChemComp-PX2: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)