[English] 日本語
 Yorodumi
Yorodumi- EMDB-34585: Molecular recognition of two endogenous hormones by the human par... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Molecular recognition of two endogenous hormones by the human parathyroid hormone receptor-1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | PTH1R / GPCR / LIPID BINDING PROTEIN-HORMONE-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationparathyroid hormone receptor binding / type 1 parathyroid hormone receptor binding / negative regulation of bone mineralization involved in bone maturation / positive regulation of osteoclast proliferation / negative regulation of apoptotic process in bone marrow cell / response to parathyroid hormone / positive regulation of cell proliferation in bone marrow / macromolecule biosynthetic process / parathyroid hormone receptor activity / hormone-mediated apoptotic signaling pathway ...parathyroid hormone receptor binding / type 1 parathyroid hormone receptor binding / negative regulation of bone mineralization involved in bone maturation / positive regulation of osteoclast proliferation / negative regulation of apoptotic process in bone marrow cell / response to parathyroid hormone / positive regulation of cell proliferation in bone marrow / macromolecule biosynthetic process / parathyroid hormone receptor activity / hormone-mediated apoptotic signaling pathway / magnesium ion homeostasis / positive regulation of signal transduction / response to fibroblast growth factor / phosphate ion homeostasis / cAMP metabolic process / response to vitamin D / G-protein activation / Activation of the phototransduction cascade / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Ca2+ pathway / G alpha (z) signalling events / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Class B/2 (Secretin family receptors) / G protein-coupled peptide receptor activity / Adrenaline,noradrenaline inhibits insulin secretion / ADP signalling through P2Y purinoceptor 12 / G alpha (q) signalling events / negative regulation of chondrocyte differentiation / osteoblast development / G alpha (i) signalling events / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / photoreceptor outer segment membrane / G alpha (q) signalling events / peptide hormone receptor binding / positive regulation of inositol phosphate biosynthetic process / G alpha (i) signalling events / spectrin binding / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / bone mineralization / alkylglycerophosphoethanolamine phosphodiesterase activity / peptide hormone binding / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / photoreceptor outer segment / Rho protein signal transduction / chondrocyte differentiation / positive regulation of glycogen biosynthetic process / bone resorption / positive regulation of bone mineralization / response to cadmium ion / cell maturation / homeostasis of number of cells within a tissue / photoreceptor inner segment / cardiac muscle cell apoptotic process / skeletal system development / positive regulation of D-glucose import / response to lead ion / hormone activity / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway / intracellular calcium ion homeostasis / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / sensory perception of taste / cell-cell signaling / signaling receptor complex adaptor activity Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.62 Å | |||||||||
 Authors Authors | Zhao L / Xu HE / Yuan Q | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Acta Pharmacol Sin / Year: 2023 Journal: Acta Pharmacol Sin / Year: 2023Title: Molecular recognition of two endogenous hormones by the human parathyroid hormone receptor-1. Authors: Li-Hua Zhao / Qing-Ning Yuan / An-Tao Dai / Xin-Heng He / Chuan-Wei Chen / Chao Zhang / You-Wei Xu / Yan Zhou / Ming-Wei Wang / De-Hua Yang / H Eric Xu /   Abstract: Parathyroid hormone (PTH) and PTH-related peptide (PTHrP) are two endogenous hormones recognized by PTH receptor-1 (PTH1R), a member of class B G protein- coupled receptors (GPCRs). Both PTH and ...Parathyroid hormone (PTH) and PTH-related peptide (PTHrP) are two endogenous hormones recognized by PTH receptor-1 (PTH1R), a member of class B G protein- coupled receptors (GPCRs). Both PTH and PTHrP analogs including teriparatide and abaloparatide are approved drugs for osteoporosis, but they exhibit distinct pharmacology. Here we report two cryo-EM structures of human PTH1R bound to PTH and PTHrP in the G protein-bound state at resolutions of 2.62 Å and 3.25 Å, respectively. Detailed analysis of these structures uncovers both common and unique features for the agonism of PTH and PTHrP. Molecular dynamics (MD) simulation together with site-directed mutagenesis studies reveal the molecular basis of endogenous hormones recognition specificity and selectivity to PTH1R. These results provide a rational template for the clinical use of PTH and PTHrP analogs as an anabolic therapy for osteoporosis and other disorders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34585.map.gz emd_34585.map.gz | 89.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34585-v30.xml emd-34585-v30.xml emd-34585.xml emd-34585.xml | 22 KB 22 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34585.png emd_34585.png | 263.4 KB | ||
| Masks |  emd_34585_msk_1.map emd_34585_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-34585.cif.gz emd-34585.cif.gz | 6.8 KB | ||
| Others |  emd_34585_half_map_1.map.gz emd_34585_half_map_1.map.gz emd_34585_half_map_2.map.gz emd_34585_half_map_2.map.gz | 80.8 MB 80.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34585 http://ftp.pdbj.org/pub/emdb/structures/EMD-34585 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34585 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34585 | HTTPS FTP |
-Related structure data
| Related structure data |  8ha0MC  8hafC  8haoC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34585.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34585.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.824 Å | ||||||||||||||||||||||||||||||||||||
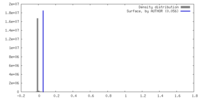
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_34585_msk_1.map emd_34585_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
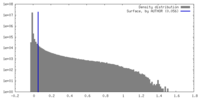
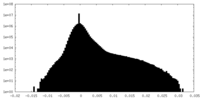
| Density Histograms |
-Half map: #2
| File | emd_34585_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
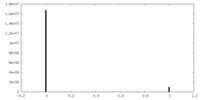
| ||||||||||||
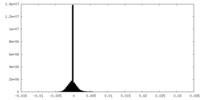
| Density Histograms |
-Half map: #1
| File | emd_34585_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
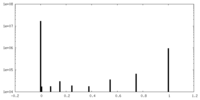
| ||||||||||||
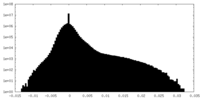
| Density Histograms |
- Sample components
Sample components
-Entire : PTH-PTHR G protein complex
| Entire | Name: PTH-PTHR G protein complex |
|---|---|
| Components |
|
-Supramolecule #1: PTH-PTHR G protein complex
| Supramolecule | Name: PTH-PTHR G protein complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein g(s) subunit alpha
| Macromolecule | Name: Guanine nucleotide-binding protein g(s) subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.879465 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RATHRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RATHRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTSGIFETK FQVDKVNFHM FDVGAQRDER RKWIQCFNDV TAIIFVVDSS DYNRLQEALN DF KSIWNNR WLRTISVILF LNKQDLLAEK VLAGKSKIED YFPEFARYTT PEDATPEPGE DPRVTRAKYF IRDEFLRIST ASG DGRHYC YPHFTCSVDT ENARRIFNDC RDIIQRMHLR QYELL |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 43.70675 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MHHHHHHSSG LVPRGSHMAS HHHHHHHHHH GSLLQSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN L KTREGNVR ...String: MHHHHHHSSG LVPRGSHMAS HHHHHHHHHH GSLLQSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN L KTREGNVR VSRELAGHTG YLSCCRFLDD NQIVTSSGDT TCALWDIETG QQTTTFTGHT GDVMSLSLAP DTRLFVSGAC DA SAKLWDV REGMCRQTFT GHESDINAIC FFPNGNAFAT GSDDATCRLF DLRADQELMT YSHDNIICGI TSVSFSKSGR LLL AGYDDF NCNVWDALKA DRAGVLAGHD NRVSCLGVTD DGMAVATGSW DSFLKIWNGS SGGGGSGGGG SSGVSGWRLF KKIS UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Nanobody 35
| Macromolecule | Name: Nanobody 35 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 15.343019 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAQVQLQESG GGLVQPGGSL RLSCAASGFT FSNYKMNWVR QAPGKGLEWV SDISQSGASI SYTGSVKGRF TISRDNAKNT LYLQMNSLK PEDTAVYYCA RCPAPFTRDC FDVTSTTYAY RGQGTQVTVS SHHHHHHEPE A |
-Macromolecule #5: Parathyroid hormone
| Macromolecule | Name: Parathyroid hormone / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 4.125778 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: SVSEIQLMHN LGKHLNSMER VEWLRKKLQD VHNF UniProtKB: Parathyroid hormone |
-Macromolecule #6: Parathyroid hormone/parathyroid hormone-related peptide receptor
| Macromolecule | Name: Parathyroid hormone/parathyroid hormone-related peptide receptor type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 54.506734 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: DADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASGK LYPESEEDKE APTGSRYRGR PCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE LVPGHNRTWA NYSECVKFLT NETREREVFD R LAMIYTVG ...String: DADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASGK LYPESEEDKE APTGSRYRGR PCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE LVPGHNRTWA NYSECVKFLT NETREREVFD R LAMIYTVG YSVSLASLTV AVLILAYFRR LHCTRNYIHM HLFLSFMLRA VSIFVKDAVL YSGATLDEAE RLTEEELRAI AQ APPPPAT AAAGYAGCRV AVTFFLYFLA TNYYWILVEG LYLHSLIFMA FFSEKKYLWG FTVFGWGLPA VFVAVWVSVR ATL ANTGCW DLSSGNKKWI IQVPILASIV LNFILFINIV RVLATKLRET NAGRCDTRQQ YRKLLKSTLV LMPLFGVHYI VFMA TPYTE VSGTLWQVQM HYEMLFNSFQ GFFVAIIYCF CNGEVQAEIK KSWSRWTLAL DFRRKARSGS SSYSYGPMVS H UniProtKB: Parathyroid hormone/parathyroid hormone-related peptide receptor |
-Macromolecule #7: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 7 / Number of copies: 4 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #8: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 8 / Number of copies: 3 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 89.0 K / Max: 100.0 K |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 2.2 µm / Calibrated defocus min: 0.6 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.62 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 407467 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)