+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
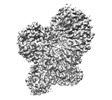
| Title | Structure of mouse SCMC bound with KH domain of FILIA | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | oocyte / subcortical / complex / CYTOSOLIC PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsubcortical maternal complex / establishment of organelle localization / protein storage / structural constituent of cytoplasmic lattice / cytoplasmic lattice / cortical granule exocytosis / endoplasmic reticulum localization / establishment or maintenance of apical/basal cell polarity / ooplasm / spermatogonial cell division ...subcortical maternal complex / establishment of organelle localization / protein storage / structural constituent of cytoplasmic lattice / cytoplasmic lattice / cortical granule exocytosis / endoplasmic reticulum localization / establishment or maintenance of apical/basal cell polarity / ooplasm / spermatogonial cell division / cortical granule / positive regulation of meiotic nuclear division / apical cortex / regulation of RNA stability / positive regulation of embryonic development / regulation of establishment of protein localization / embryonic pattern specification / flagellated sperm motility / mitochondrion localization / establishment of spindle localization / fertilization / epigenetic programming in the zygotic pronuclei / positive regulation of neurogenesis / positive regulation of double-strand break repair / exocytosis / replication fork processing / regulation of cell division / positive regulation of double-strand break repair via homologous recombination / positive regulation of neuron differentiation / embryo implantation / actin filament organization / animal organ morphogenesis / regulation of protein stability / apical part of cell / intracellular protein localization / sperm midpiece / regulation of protein localization / protein-containing complex assembly / cell cortex / spermatogenesis / in utero embryonic development / nucleolus / negative regulation of transcription by RNA polymerase II / Golgi apparatus / protein-containing complex / mitochondrion / RNA binding / ATP binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Chi P / Ou G / Han Z / Li J / Deng D | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structural basis of the subcortical maternal complex and its implications in reproductive disorders. Authors: Pengliang Chi / Guojin Ou / Dandan Qin / Zhuo Han / Jialu Li / Qingjie Xiao / Zheng Gao / Chengpeng Xu / Qianqian Qi / Qingting Liu / Sibei Liu / Jinhong Li / Li Guo / Yuechao Lu / Jing Chen ...Authors: Pengliang Chi / Guojin Ou / Dandan Qin / Zhuo Han / Jialu Li / Qingjie Xiao / Zheng Gao / Chengpeng Xu / Qianqian Qi / Qingting Liu / Sibei Liu / Jinhong Li / Li Guo / Yuechao Lu / Jing Chen / Xiang Wang / Hubing Shi / Lei Li / Dong Deng /  Abstract: The subcortical maternal complex (SCMC) plays a crucial role in early embryonic development. Malfunction of SCMC leads to reproductive diseases in women. However, the molecular function and assembly ...The subcortical maternal complex (SCMC) plays a crucial role in early embryonic development. Malfunction of SCMC leads to reproductive diseases in women. However, the molecular function and assembly basis for SCMC remain elusive. Here we reconstituted mouse SCMC and solved the structure at atomic resolution using single-particle cryo-electron microscopy. The core complex of SCMC was formed by MATER, TLE6 and FLOPED, and MATER embraced TLE6 and FLOPED via its NACHT and LRR domains. Two core complexes further dimerize through interactions between two LRR domains of MATERs in vitro. FILIA integrates into SCMC by interacting with the carboxyl-terminal region of FLOPED. Zygotes from mice with Floped C-terminus truncation showed delayed development and resembled the phenotype of zygotes from Filia knockout mice. More importantly, the assembly of mouse SCMC was affected by corresponding clinical variants associated with female reproductive diseases and corresponded with a prediction based on the mouse SCMC structure. Our study paves the way for further investigations on SCMC functions during mammalian preimplantation embryonic development and reveals underlying causes of female reproductive diseases related to SCMC mutations, providing a new strategy for the diagnosis of female reproductive disorders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34554.map.gz emd_34554.map.gz | 21 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34554-v30.xml emd-34554-v30.xml emd-34554.xml emd-34554.xml | 20.5 KB 20.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34554.png emd_34554.png | 102.3 KB | ||
| Filedesc metadata |  emd-34554.cif.gz emd-34554.cif.gz | 7.2 KB | ||
| Others |  emd_34554_half_map_1.map.gz emd_34554_half_map_1.map.gz emd_34554_half_map_2.map.gz emd_34554_half_map_2.map.gz | 20.6 MB 20.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34554 http://ftp.pdbj.org/pub/emdb/structures/EMD-34554 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34554 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34554 | HTTPS FTP |
-Related structure data
| Related structure data |  8h94MC  8h93C  8h95C  8h96C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34554.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34554.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34554_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
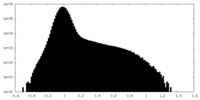
| Projections & Slices |
| ||||||||||||
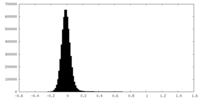
| Density Histograms |
-Half map: #1
| File | emd_34554_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
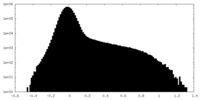
| Projections & Slices |
| ||||||||||||
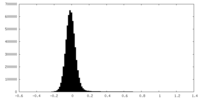
| Density Histograms |
- Sample components
Sample components
-Entire : mouse SCMC bound with KH domain of FILIA
| Entire | Name: mouse SCMC bound with KH domain of FILIA |
|---|---|
| Components |
|
-Supramolecule #1: mouse SCMC bound with KH domain of FILIA
| Supramolecule | Name: mouse SCMC bound with KH domain of FILIA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: NACHT, LRR and PYD domains-containing protein 5
| Macromolecule | Name: NACHT, LRR and PYD domains-containing protein 5 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 119.819859 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGPPEKDSKA ILKARGLEEE QKSESTMSPS ENVSRAILKD SGSEEVEQAS ERKMTSPEND SKSIQKDQGP EQEQTSETLQ SKEEDEVTE ADKDNGGDLQ DYKAHVIAKF DTSVDLHYDS PEMKLLSDAF KPYQKTFQPH TIILHGRPGV GKSALARSIV L GWAQGKLF ...String: MGPPEKDSKA ILKARGLEEE QKSESTMSPS ENVSRAILKD SGSEEVEQAS ERKMTSPEND SKSIQKDQGP EQEQTSETLQ SKEEDEVTE ADKDNGGDLQ DYKAHVIAKF DTSVDLHYDS PEMKLLSDAF KPYQKTFQPH TIILHGRPGV GKSALARSIV L GWAQGKLF QKMSFVIFFS VREIKWTEKS SLAQLIAKEC PDSWDLVTKI MSQPERLLFV IDGLDDMDSV LQHDDMTLSR DW KDEQPIY ILMYSLLRKA LLPQSFLIIT TRNTGLEKLK SMVVSPLYIL VEGLSASRRS QLVLENISNE SDRIQVFHSL IEN HQLFDQ CQAPSVCSLV CEALQLQKKL GKRCTLPCQT LTGLYATLVF HQLTLKRPSQ SALSQEEQIT LVGLCMMAAE GVWT MRSVF YDDDLKNYSL KESEILALFH MNILLQVGHN SEQCYVFSHL SLQDFFAALY YVLEGLEEWN QHFCFIENQR SIMEV KRTD DTRLLGMKRF LFGLMNKDIL KTLEVLFEYP VIPTVEQKLQ HWVSLIAQQV NGTSPMDTLD AFYCLFESQD EEFVGG ALK RFQEVWLLIN QKMDLKVSSY CLKHCQNLKA IRVDIRDLLS VDNTLELCPV VTVQETQCKP LLMEWWGNFC SVLGSLR NL KELDLGDSIL SQRAMKILCL ELRNQSCRIQ KLTFKSAEVV SGLKHLWKLL FSNQNLKYLN LGNTPMKDDD MKLACEAL K HPKCSVETLR LDSCELTIIG YEMISTLLIS TTRLKCLSLA KNRVGVKSMI SLGNALSSSM CLLQKLILDN CGLTPASCH LLVSALFSNQ NLTHLCLSNN SLGTEGVQQL CQFLRNPECA LQRLILNHCN IVDDAYGFLA MRLANNTKLT HLSLTMNPVG DGAMKLLCE ALKEPTCYLQ ELELVDCQLT QNCCEDLACM ITTTKHLKSL DLGNNALGDK GVITLCEGLK QSSSSLRRLG L GACKLTSN CCEALSLAIS CNPHLNSLNL VKNDFSTSGM LKLCSAFQCP VSNLGIIGLW KQEYYARVRR QLEEVEFVKP HV VIDGDWY ASDEDDRNWW KN UniProtKB: NACHT, LRR and PYD domains-containing protein 5 |
-Macromolecule #2: Transducin-like enhancer protein 6
| Macromolecule | Name: Transducin-like enhancer protein 6 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.187332 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTSHRQSSDT FGGILPSTLS SRYLSIVNQL PEEFSSVVSE MMVHLENIFS LAENFFQAIE RFSRTPDLLE RNKMSIGVGA EGDSWPCHV SHEAPMGSAQ TTENSAKEED KQVPESAALQ HPKFKSTPGP QLPTRRRFLS ESDELQDPQP VWDAEPQFCQ G FLIQGLWE ...String: MTSHRQSSDT FGGILPSTLS SRYLSIVNQL PEEFSSVVSE MMVHLENIFS LAENFFQAIE RFSRTPDLLE RNKMSIGVGA EGDSWPCHV SHEAPMGSAQ TTENSAKEED KQVPESAALQ HPKFKSTPGP QLPTRRRFLS ESDELQDPQP VWDAEPQFCQ G FLIQGLWE LFMDSRQKNQ QEHGGEDSSQ ESKDSGLCDF KPEPQPRHRN SLSDSADPFL IKSPSALLDY YQEDVSRPQP ET QESSGRA DKFLKPLSWG SEVLESSCNQ PSTALWQLER FTVPQALQKV RVLKHQELLL VVAVSSFTRH VFTCSQSGIK VWN LVNQVA EDRDPESHLK CSVQDNKVYL RTCLLSSNSR TLFAGGYNLP GVIVWDLAAP SLYEKCQLPC EGLSCQALAN TKEN MALAG FTDGTVRIWD LRTQEIVRNL KGPTNSARNL VVKDDNIWTG GLDACLRCWD LRMAKVSLEH LFQSQIMSLA HSPTE DWLL LGLANGQHCL FNSRKRDQVL TVDTKDNTIL GLKFSPNGKW WASVGMGNFI TVHSMPTGAK LFQVPEVGPV RCFDMT ENG RLIITGSRDC ASVYHIKY UniProtKB: Transducin-like enhancer protein 6 |
-Macromolecule #3: Oocyte-expressed protein homolog
| Macromolecule | Name: Oocyte-expressed protein homolog / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 18.481295 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASHTADADA KPDSDSQKLL NVLPVSLRLR TRPWWFPIQE VSNPLVLYME AWVAERVIGT DQAEISEIEW MCQALLTVDS VNSGNLAEI TIFGQPSAQT RMKNILLNMA AWHKENELQR AVKVKEVEEF LKIRASSILS KLSKKGLKLA GFPLPLEGRE T QMES UniProtKB: Oocyte-expressed protein homolog |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 46.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.7 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)