[English] 日本語
 Yorodumi
Yorodumi- EMDB-34272: Cryo-EM structure of cancer-specific PI3Kalpha mutant H1047R in c... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of cancer-specific PI3Kalpha mutant H1047R in complex with BYL-719 | ||||||||||||||||||||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | Phosphoinositide 3-kinase (PI3K) / kinase domain / mutation / cancers / STRUCTURAL PROTEIN | ||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationperinuclear endoplasmic reticulum membrane / regulation of toll-like receptor 4 signaling pathway / response to muscle inactivity / phosphatidylinositol kinase activity / regulation of actin filament organization / negative regulation of actin filament depolymerization / positive regulation of focal adhesion disassembly / response to butyrate / response to L-leucine / 1-phosphatidylinositol-3-kinase regulator activity ...perinuclear endoplasmic reticulum membrane / regulation of toll-like receptor 4 signaling pathway / response to muscle inactivity / phosphatidylinositol kinase activity / regulation of actin filament organization / negative regulation of actin filament depolymerization / positive regulation of focal adhesion disassembly / response to butyrate / response to L-leucine / 1-phosphatidylinositol-3-kinase regulator activity / phosphatidylinositol 3-kinase regulator activity / IRS-mediated signalling / positive regulation of endoplasmic reticulum unfolded protein response / phosphatidylinositol 3-kinase activator activity / interleukin-18-mediated signaling pathway / T follicular helper cell differentiation / phosphatidylinositol 3-kinase complex / PI3K events in ERBB4 signaling / phosphatidylinositol 3-kinase regulatory subunit binding / autosome genomic imprinting / cellular response to hydrostatic pressure / myeloid leukocyte migration / neurotrophin TRKA receptor binding / Activated NTRK2 signals through PI3K / regulation of cellular respiration / cis-Golgi network / negative regulation of fibroblast apoptotic process / transmembrane receptor protein tyrosine kinase adaptor activity / Activated NTRK3 signals through PI3K / phosphatidylinositol 3-kinase complex, class IB / ErbB-3 class receptor binding / vasculature development / positive regulation of protein localization to membrane / 1-phosphatidylinositol-4-phosphate 3-kinase activity / Signaling by cytosolic FGFR1 fusion mutants / Co-stimulation by ICOS / RHOD GTPase cycle / cardiac muscle cell contraction / phosphatidylinositol 3-kinase complex, class IA / Nephrin family interactions / RHOF GTPase cycle / Signaling by LTK in cancer / anoikis / kinase activator activity / phosphatidylinositol-3-phosphate biosynthetic process / Signaling by LTK / positive regulation of leukocyte migration / MET activates PI3K/AKT signaling / relaxation of cardiac muscle / PI3K/AKT activation / 1-phosphatidylinositol-4,5-bisphosphate 3-kinase activity / RND1 GTPase cycle / RND2 GTPase cycle / phosphatidylinositol-4,5-bisphosphate 3-kinase / negative regulation of stress fiber assembly / RND3 GTPase cycle / positive regulation of filopodium assembly / vascular endothelial growth factor signaling pathway / phosphatidylinositol 3-kinase / growth hormone receptor signaling pathway / insulin binding / Signaling by ALK / 1-phosphatidylinositol-3-kinase activity / RHOV GTPase cycle / Erythropoietin activates Phosphoinositide-3-kinase (PI3K) / RHOB GTPase cycle / PI-3K cascade:FGFR3 / natural killer cell mediated cytotoxicity / GP1b-IX-V activation signalling / negative regulation of macroautophagy / response to dexamethasone / PI-3K cascade:FGFR2 / phosphatidylinositol-mediated signaling / PI-3K cascade:FGFR4 / PI-3K cascade:FGFR1 / RHOC GTPase cycle / RHOJ GTPase cycle / intracellular glucose homeostasis / phosphatidylinositol phosphate biosynthetic process / negative regulation of osteoclast differentiation / Synthesis of PIPs at the plasma membrane / RHOU GTPase cycle / CDC42 GTPase cycle / RET signaling / negative regulation of anoikis / insulin receptor substrate binding / Interleukin-3, Interleukin-5 and GM-CSF signaling / PI3K events in ERBB2 signaling / intercalated disc / PI3K Cascade / T cell differentiation / negative regulation of cell-matrix adhesion / RHOG GTPase cycle / extrinsic apoptotic signaling pathway via death domain receptors / regulation of multicellular organism growth / CD28 dependent PI3K/Akt signaling / Role of LAT2/NTAL/LAB on calcium mobilization / RHOA GTPase cycle / RAC2 GTPase cycle / RAC3 GTPase cycle Similarity search - Function | ||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.73 Å | ||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Liu X / Zhou Q / Hart JR / Xu Y / Yang S / Yang D / Vogt PK / Wang M-W | ||||||||||||||||||||||||||||||||||||||||||
| Funding support |  China, China,  United States, 13 items United States, 13 items
| ||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: Cryo-EM structures of cancer-specific helical and kinase domain mutations of PI3Kα. Authors: Xiao Liu / Qingtong Zhou / Jonathan R Hart / Yingna Xu / Su Yang / Dehua Yang / Peter K Vogt / Ming-Wei Wang /    Abstract: Phosphoinositide 3-kinases (PI3Ks) are a family of lipid kinases that perform multiple and important cellular functions. The protein investigated here belongs to class IA of the PI3Ks; it is a dimer ...Phosphoinositide 3-kinases (PI3Ks) are a family of lipid kinases that perform multiple and important cellular functions. The protein investigated here belongs to class IA of the PI3Ks; it is a dimer consisting of a catalytic subunit, p110α, and a regulatory subunit, p85α, and is referred to as PI3Kα. The catalytic subunit p110α is frequently mutated in cancer. The mutations induce a gain of function and constitute a driving force in cancer development. About 80% of these mutations lead to single-amino-acid substitutions in one of three sites of p110α: two in the helical domain of the protein (E542K and E545K) and one at the C-terminus of the kinase domain (H1047R). Here, we report the cryo-electron microscopy structures of these mutants in complex with the p110α-specific inhibitor BYL-719. The H1047R mutant rotates its sidechain to a new position and weakens the kα11 activation loop interaction, thereby reducing the inhibitory effect of p85α on p110α. E542K and E545K completely abolish the tight interaction between the helical domain of p110α and the N-terminal SH2 domain of p85α and lead to the disruption of all p85α binding and a dramatic increase in flexibility of the adaptor-binding domain (ABD) in p110α. Yet, the dimerization of PI3Kα is preserved through the ABD-p85α interaction. The local and global structural features induced by these mutations provide molecular insights into the activation of PI3Kα, deepen our understanding of the oncogenic mechanism of this important signaling molecule, and may facilitate the development of mutant-specific inhibitors. | ||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34272.map.gz emd_34272.map.gz | 59.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34272-v30.xml emd-34272-v30.xml emd-34272.xml emd-34272.xml | 19.8 KB 19.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_34272_fsc.xml emd_34272_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_34272.png emd_34272.png | 96.3 KB | ||
| Filedesc metadata |  emd-34272.cif.gz emd-34272.cif.gz | 7.1 KB | ||
| Others |  emd_34272_half_map_1.map.gz emd_34272_half_map_1.map.gz emd_34272_half_map_2.map.gz emd_34272_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34272 http://ftp.pdbj.org/pub/emdb/structures/EMD-34272 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34272 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34272 | HTTPS FTP |
-Related structure data
| Related structure data |  8gubMC  8guaC  8gudC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34272.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34272.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34272_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
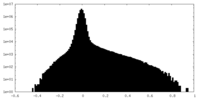
| Density Histograms |
-Half map: #1
| File | emd_34272_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
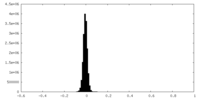
| Density Histograms |
- Sample components
Sample components
-Entire : Human PI3Kalpha mutant H1047R in complex with BYL-719
| Entire | Name: Human PI3Kalpha mutant H1047R in complex with BYL-719 |
|---|---|
| Components |
|
-Supramolecule #1: Human PI3Kalpha mutant H1047R in complex with BYL-719
| Supramolecule | Name: Human PI3Kalpha mutant H1047R in complex with BYL-719 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit ...
| Macromolecule | Name: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: phosphatidylinositol 3-kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 127.841625 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MSYYHHHHHH DYDIPTTENL YFQGAMGSMP PRPSSGELWG IHLMPPRILV ECLLPNGMIV TLECLREATL ITIKHELFKE ARKYPLHQL LQDESSYIFV SVTQEAEREE FFDETRRLCD LRLFQPFLKV IEPVGNREEK ILNREIGFAI GMPVCEFDMV K DPEVQDFR ...String: MSYYHHHHHH DYDIPTTENL YFQGAMGSMP PRPSSGELWG IHLMPPRILV ECLLPNGMIV TLECLREATL ITIKHELFKE ARKYPLHQL LQDESSYIFV SVTQEAEREE FFDETRRLCD LRLFQPFLKV IEPVGNREEK ILNREIGFAI GMPVCEFDMV K DPEVQDFR RNILNVCKEA VDLRDLNSPH SRAMYVYPPN VESSPELPKH IYNKLDKGQI IVVIWVIVSP NNDKQKYTLK IN HDCVPEQ VIAEAIRKKT RSMLLSSEQL KLCVLEYQGK YILKVCGCDE YFLEKYPLSQ YKYIRSCIML GRMPNLMLMA KES LYSQLP MDCFTMPSYS RRISTATPYM NGETSTKSLW VINSALRIKI LCATYVNVNI RDIDKIYVRT GIYHGGEPLC DNVN TQRVP CSNPRWNEWL NYDIYIPDLP RAARLCLSIC SVKGRKGAKE EHCPLAWGNI NLFDYTDTLV SGKMALNLWP VPHGL EDLL NPIGVTGSNP NKETPCLELE FDWFSSVVKF PDMSVIEEHA NWSVSREAGF SYSHAGLSNR LARDNELREN DKEQLK AIS TRDPLSEITE QEKDFLWSHR HYCVTIPEIL PKLLLSVKWN SRDEVAQMYC LVKDWPPIKP EQAMELLDCN YPDPMVR GF AVRCLEKYLT DDKLSQYLIQ LVQVLKYEQY LDNLLVRFLL KKALTNQRIG HFFFWHLKSE MHNKTVSQRF GLLLESYC R ACGMYLKHLN RQVEAMEKLI NLTDILKQEK KDETQKVQMK FLVEQMRRPD FMDALQGFLS PLNPAHQLGN LRLEECRIM SSAKRPLWLN WENPDIMSEL LFQNNEIIFK NGDDLRQDML TLQIIRIMEN IWQNQGLDLR MLPYGCLSIG DCVGLIEVVR NSHTIMQIQ CKGGLKGALQ FNSHTLHQWL KDKNKGEIYD AAIDLFTRSC AGYCVATFIL GIGDRHNSNI MVKDDGQLFH I DFGHFLDH KKKKFGYKRE RVPFVLTQDF LIVISKGAQE CTKTREFERF QEMCYKAYLA IRQHANLFIN LFSMMLGSGM PE LQSFDDI AYIRKTLALD KTEQEALEYF MKQMNDARHG GWTTKMDWIF HTIKQHALN UniProtKB: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform |
-Macromolecule #2: Phosphatidylinositol 3-kinase regulatory subunit alpha
| Macromolecule | Name: Phosphatidylinositol 3-kinase regulatory subunit alpha type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 83.623203 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MAEGYQYRAL YDYKKEREED IDLHLGDILT VNKGSLVALG FSDGQEARPE EIGWLNGYNE TTGERGDFPG TYVEYIGRKK ISPPTPKPR PPRPLPVAPG SSKTEADVEQ QALTLPDLAE QFAPPDIAPP LLIKLVEAIE KKGLECSTLY RTQSSSNLAE L RQLLDCDT ...String: MAEGYQYRAL YDYKKEREED IDLHLGDILT VNKGSLVALG FSDGQEARPE EIGWLNGYNE TTGERGDFPG TYVEYIGRKK ISPPTPKPR PPRPLPVAPG SSKTEADVEQ QALTLPDLAE QFAPPDIAPP LLIKLVEAIE KKGLECSTLY RTQSSSNLAE L RQLLDCDT PSVDLEMIDV HVLADAFKRY LLDLPNPVIP AAVYSEMISL APEVQSSEEY IQLLKKLIRS PSIPHQYWLT LQ YLLKHFF KLSQTSSKNL LNARVLSEIF SPMLFRFSAA SSDNTENLIK VIEILISTEW NERQPAPALP PKPPKPTTVA NNG MNNNMS LQDAEWYWGD ISREEVNEKL RDTADGTFLV RDASTKMHGD YTLTLRKGGN NKLIKIFHRD GKYGFSDPLT FSSV VELIN HYRNESLAQY NPKLDVKLLY PVSKYQQDQV VKEDNIEAVG KKLHEYNTQF QEKSREYDRL YEEYTRTSQE IQMKR TAIE AFNETIKIFE EQCQTQERYS KEYIEKFKRE GNEKEIQRIM HNYDKLKSRI SEIIDSRRRL EEDLKKQAAE YREIDK RMN SIKPDLIQLR KTRDQYLMWL TQKGVRQKKL NEWLGNENTE DQYSLVEDDE DLPHHDEKTW NVGSSNRNKA ENLLRGK RD GTFLVRESSK QGCYACSVVV DGEVKHCVIN KTATGYGFAE PYNLYSSLKE LVLHYQHTSL VQHNDSLNVT LAYPVYAQ Q RR UniProtKB: Phosphatidylinositol 3-kinase regulatory subunit alpha |
-Macromolecule #3: (2S)-N~1~-{4-methyl-5-[2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyr...
| Macromolecule | Name: (2S)-N~1~-{4-methyl-5-[2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin-4-yl]-1,3-thiazol-2-yl}pyrrolidine-1,2-dicarboxamide type: ligand / ID: 3 / Number of copies: 1 / Formula: 1LT |
|---|---|
| Molecular weight | Theoretical: 441.47 Da |
| Chemical component information | 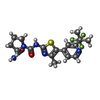 ChemComp-1LT: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 7.6 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 5881 / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source: OTHER |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 89.2 / Target criteria: Correlation coefficient |
| Output model |  PDB-8gub: |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)