+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | human TMEM63A | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | channel / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsurfactant secretion / osmolarity-sensing monoatomic cation channel activity / mechanosensitive monoatomic ion channel activity / calcium-activated cation channel activity / lysosome organization / tertiary granule membrane / specific granule membrane / centriolar satellite / early endosome membrane / nucleic acid binding ...surfactant secretion / osmolarity-sensing monoatomic cation channel activity / mechanosensitive monoatomic ion channel activity / calcium-activated cation channel activity / lysosome organization / tertiary granule membrane / specific granule membrane / centriolar satellite / early endosome membrane / nucleic acid binding / lysosomal membrane / intracellular membrane-bounded organelle / Neutrophil degranulation / extracellular exosome / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Zhang MF | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: A mechanical-coupling mechanism in OSCA/TMEM63 channel mechanosensitivity. Authors: Mingfeng Zhang / Yuanyue Shan / Charles D Cox / Duanqing Pei /   Abstract: Mechanosensitive (MS) ion channels are a ubiquitous type of molecular force sensor sensing forces from the surrounding bilayer. The profound structural diversity in these channels suggests that the ...Mechanosensitive (MS) ion channels are a ubiquitous type of molecular force sensor sensing forces from the surrounding bilayer. The profound structural diversity in these channels suggests that the molecular mechanisms of force sensing follow unique structural blueprints. Here we determine the structures of plant and mammalian OSCA/TMEM63 proteins, allowing us to identify essential elements for mechanotransduction and propose roles for putative bound lipids in OSCA/TMEM63 mechanosensation. Briefly, the central cavity created by the dimer interface couples each subunit and modulates dimeric OSCA/TMEM63 channel mechanosensitivity through the modulating lipids while the cytosolic side of the pore is gated by a plug lipid that prevents the ion permeation. Our results suggest that the gating mechanism of OSCA/TMEM63 channels may combine structural aspects of the 'lipid-gated' mechanism of MscS and TRAAK channels and the calcium-induced gating mechanism of the TMEM16 family, which may provide insights into the structural rearrangements of TMEM16/TMC superfamilies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34214.map.gz emd_34214.map.gz | 117.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34214-v30.xml emd-34214-v30.xml emd-34214.xml emd-34214.xml | 13.5 KB 13.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34214.png emd_34214.png | 34.2 KB | ||
| Filedesc metadata |  emd-34214.cif.gz emd-34214.cif.gz | 5.5 KB | ||
| Others |  emd_34214_half_map_1.map.gz emd_34214_half_map_1.map.gz emd_34214_half_map_2.map.gz emd_34214_half_map_2.map.gz | 116.1 MB 116.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34214 http://ftp.pdbj.org/pub/emdb/structures/EMD-34214 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34214 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34214 | HTTPS FTP |
-Validation report
| Summary document |  emd_34214_validation.pdf.gz emd_34214_validation.pdf.gz | 1006.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34214_full_validation.pdf.gz emd_34214_full_validation.pdf.gz | 1005.9 KB | Display | |
| Data in XML |  emd_34214_validation.xml.gz emd_34214_validation.xml.gz | 13.9 KB | Display | |
| Data in CIF |  emd_34214_validation.cif.gz emd_34214_validation.cif.gz | 16.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34214 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34214 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34214 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34214 | HTTPS FTP |
-Related structure data
| Related structure data |  8grsMC  8grnC  8groC  8gsoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_34214.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34214.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
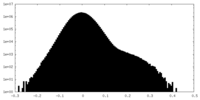
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||
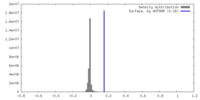
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34214_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
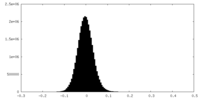
| Density Histograms |
-Half map: #1
| File | emd_34214_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
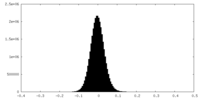
| Density Histograms |
- Sample components
Sample components
-Entire : human TMEM63A
| Entire | Name: human TMEM63A |
|---|---|
| Components |
|
-Supramolecule #1: human TMEM63A
| Supramolecule | Name: human TMEM63A / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: CSC1-like protein 1
| Macromolecule | Name: CSC1-like protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 92.212836 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MMDSPFLELW QSKAVSIREQ LGLGDRPNDS YCYNSAKNST VLQGVTFGGI PTVLLIDVSC FLFLILVFSI IRRRFWDYGR IALVSEADS ESRFQRLSST SSSGQQDFEN ELGCCPWLTA IFRLHDDQIL EWCGEDAIHY LSFQRHIIFL LVVVSFLSLC V ILPVNLSG ...String: MMDSPFLELW QSKAVSIREQ LGLGDRPNDS YCYNSAKNST VLQGVTFGGI PTVLLIDVSC FLFLILVFSI IRRRFWDYGR IALVSEADS ESRFQRLSST SSSGQQDFEN ELGCCPWLTA IFRLHDDQIL EWCGEDAIHY LSFQRHIIFL LVVVSFLSLC V ILPVNLSG DLLDKDPYSF GRTTIANLQT DNDLLWLHTI FAVIYLFLTV GFMRHHTQSI KYKEENLVRR TLFITGLPRD AR KETVESH FRDAYPTCEV VDVQLCYNVA KLIYLCKEKK KTEKSLTYYT NLQVKTGQRT LINPKPCGQF CCCEVLGCEW EDA ISYYTR MKDRLLERIT EEERHVQDQP LGMAFVTFQE KSMATYILKD FNACKCQSLQ CKGEPQPSSH SRELYTSKWT VTFA ADPED ICWKNLSIQG LRWWLQWLGI NFTLFLGLFF LTTPSIILST MDKFNVTKPI HALNNPIISQ FFPTLLLWSF SALLP SIVY YSTLLESHWT KSGENQIMMT KVYIFLIFMV LILPSLGLTS LDFFFRWLFD KTSSEASIRL ECVFLPDQGA FFVNYV IAS AFIGNGMELL RLPGLILYTF RMIMAKTAAD RRNVKQNQAF QYEFGAMYAW MLCVFTVIVA YSITCPIIAP FGLIYIL LK HMVDRHNLYF VYLPAKLEKG IHFAAVNQAL AAPILCLFWL YFFSFLRLGM KAPATLFTFL VLLLTILVCL AHTCFGCF K HLSPLNYKTE EPASDKGSEA EAHMPPPFTP YVPRILNGLA SERTALSPQQ QQQQTYGAIH NISGTIPGQC LAQSATGSV AAAPQEA UniProtKB: Mechanosensitive cation channel TMEM63A |
-Macromolecule #2: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 2 / Number of copies: 1 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 2.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 155000 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)