+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Omicron BA.4/5 SARS-CoV-2 S RBD in complex with TH132 Fab | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | COVID-19 / spike glycoprotein / virus / VIRAL PROTEIN / ANTIVIRAL PROTEIN-VIRAL PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Guo Y / Zhang G / Liang J / Liu F / Rao Z | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Discovery and characterization of potent pan-variant SARS-CoV-2 neutralizing antibodies from individuals with Omicron breakthrough infection. Authors: Yu Guo / Guangshun Zhang / Qi Yang / Xiaowei Xie / Yang Lu / Xuelian Cheng / Hui Wang / Jingxi Liang / Jielin Tang / Yuxin Gao / Hang Shang / Jun Dai / Yongxia Shi / Jiaxi Zhou / Jun Zhou / ...Authors: Yu Guo / Guangshun Zhang / Qi Yang / Xiaowei Xie / Yang Lu / Xuelian Cheng / Hui Wang / Jingxi Liang / Jielin Tang / Yuxin Gao / Hang Shang / Jun Dai / Yongxia Shi / Jiaxi Zhou / Jun Zhou / Hangtian Guo / Haitao Yang / Jianwei Qi / Lijun Liu / Shihui Ma / Biao Zhang / Qianyu Huo / Yi Xie / Junping Wu / Fang Dong / Song Zhang / Zhiyong Lou / Yan Gao / Zidan Song / Wenming Wang / Zixian Sun / Xiaoming Yang / Dongsheng Xiong / Fengjiang Liu / Xinwen Chen / Ping Zhu / Ximo Wang / Tao Cheng / Zihe Rao /  Abstract: The SARS-CoV-2 Omicron variant evades most currently approved neutralizing antibodies (nAbs) and caused drastic decrease of plasma neutralizing activity elicited by vaccination or prior infection, ...The SARS-CoV-2 Omicron variant evades most currently approved neutralizing antibodies (nAbs) and caused drastic decrease of plasma neutralizing activity elicited by vaccination or prior infection, urging the need for the development of pan-variant antivirals. Breakthrough infection induces a hybrid immunological response with potentially broad, potent and durable protection against variants, therefore, convalescent plasma from breakthrough infection may provide a broadened repertoire for identifying elite nAbs. We performed single-cell RNA sequencing (scRNA-seq) and BCR sequencing (scBCR-seq) of B cells from BA.1 breakthrough-infected patients who received 2 or 3 previous doses of inactivated vaccine. Elite nAbs, mainly derived from the IGHV2-5 and IGHV3-66/53 germlines, showed potent neutralizing activity across Wuhan-Hu-1, Delta, Omicron sublineages BA.1 and BA.2 at picomolar NT values. Cryo-EM analysis revealed diverse modes of spike recognition and guides the design of cocktail therapy. A single injection of paired antibodies cocktail provided potent protection in the K18-hACE2 transgenic female mouse model of SARS-CoV-2 infection. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34127.map.gz emd_34127.map.gz | 483.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34127-v30.xml emd-34127-v30.xml emd-34127.xml emd-34127.xml | 22.1 KB 22.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34127.png emd_34127.png | 20.3 KB | ||
| Masks |  emd_34127_msk_1.map emd_34127_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-34127.cif.gz emd-34127.cif.gz | 7.1 KB | ||
| Others |  emd_34127_half_map_1.map.gz emd_34127_half_map_1.map.gz emd_34127_half_map_2.map.gz emd_34127_half_map_2.map.gz | 474.9 MB 474.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34127 http://ftp.pdbj.org/pub/emdb/structures/EMD-34127 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34127 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34127 | HTTPS FTP |
-Validation report
| Summary document |  emd_34127_validation.pdf.gz emd_34127_validation.pdf.gz | 872.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34127_full_validation.pdf.gz emd_34127_full_validation.pdf.gz | 872.2 KB | Display | |
| Data in XML |  emd_34127_validation.xml.gz emd_34127_validation.xml.gz | 18.3 KB | Display | |
| Data in CIF |  emd_34127_validation.cif.gz emd_34127_validation.cif.gz | 21.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34127 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34127 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34127 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34127 | HTTPS FTP |
-Related structure data
| Related structure data |  7yvhMC  7yveC  7yvfC  7yvgC  7yviC  7yvjC  7yvkC  7yvlC  7yvmC  7yvnC  7yvoC  7yvpC  8gouC  8gpyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34127.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34127.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||
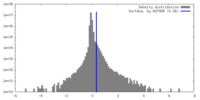
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_34127_msk_1.map emd_34127_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_34127_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_34127_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Omicron BA.4/5 SARS-CoV-2 S in complex with TH027 Fab
| Entire | Name: Omicron BA.4/5 SARS-CoV-2 S in complex with TH027 Fab |
|---|---|
| Components |
|
-Supramolecule #1: Omicron BA.4/5 SARS-CoV-2 S in complex with TH027 Fab
| Supramolecule | Name: Omicron BA.4/5 SARS-CoV-2 S in complex with TH027 Fab / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: TH027 Fab
| Supramolecule | Name: TH027 Fab / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Omicron BA.4/5 SARS-CoV-2 S
| Supramolecule | Name: Omicron BA.4/5 SARS-CoV-2 S / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: TH132 Fab light chain
| Macromolecule | Name: TH132 Fab light chain / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.785997 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: ERVMTQSPAT LSVSPGERAT LSCRASESVS SDLAWYQQKP GQAPRLLIYG ASTRATGIPA RFSGSGSGTE FTLTISSLQS EDSAVYYCQ QYNNWPPGYT FGQGTKLEIK |
-Macromolecule #2: TH132 Fab heavy chain
| Macromolecule | Name: TH132 Fab heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.638146 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EVQLVESGGG LVQPGGSLRL SCAASGITVS RNYMSWVRQA PGKGLEWVSV MFAGGSTFYA DSVKGRFTIS RDNSKNTLYL QMNSLRAED TAVYYCARDL GVVGATDYWG QGTLVTVSS |
-Macromolecule #3: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 142.592938 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFVFLVLLPL VSSQCVNLIT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLDVYYHKNN KSWMESEFRV Y SSANNCTF ...String: MFVFLVLLPL VSSQCVNLIT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLDVYYHKNN KSWMESEFRV Y SSANNCTF EYVSQPFLMD LEGKQGNFKN LREFVFKNID GYFKIYSKHT PINLGRDLPQ GFSALEPLVD LPIGINITRF QT LLALHRS YLTPGDSSSG WTAGAAAYYV GYLQPRTFLL KYNENGTITD AVDCALDPLS ETKCTLKSFT VEKGIYQTSN FRV QPTESI VRFPNITNLC PFDEVFNATR FASVYAWNRK RISNCVADYS VLYNFAPFFA FKCYGVSPTK LNDLCFTNVY ADSF VIRGN EVSQIAPGQT GNIADYNYKL PDDFTGCVIA WNSNKLDSKV GGNYNYRYRL FRKSNLKPFE RDISTEIYQA GNKPC NGVA GVNCYFPLQS YGFRPTYGVG HQPYRVVVLS FELLHAPATV CGPKKSTNLV KNKCVNFNFN GLTGTGVLTE SNKKFL PFQ QFGRDIADTT DAVRDPQTLE ILDITPCSFG GVSVITPGTN TSNQVAVLYQ GVNCTEVPVA IHADQLTPTW RVYSTGS NV FQTRAGCLIG AEYVNSSYEC DIPIGAGICA SYQTQTKSHG SASSVASQSI IAYTMSLGAE NSVAYSNNSI AIPTNFTI S VTTEILPVSM TKTSVDCTMY ICGDSTECSN LLLQYGSFCT QLKRALTGIA VEQDKNTQEV FAQVKQIYKT PPIKYFGGF NFSQILPDPS KPSKRSPIED LLFNKVTLAD AGFIKQYGDC LGDIAARDLI CAQKFNGLTV LPPLLTDEMI AQYTSALLAG TITSGWTFG AGPALQIPFP MQMAYRFNGI GVTQNVLYEN QKLIANQFNS AIGKIQDSLS STPSALGKLQ DVVNHNAQAL N TLVKQLSS KFGAISSVLN DILSRLDPPE AEVQIDRLIT GRLQSLQTYV TQQLIRAAEI RASANLAATK MSECVLGQSK RV DFCGKGY HLMSFPQSAP HGVVFLHVTY VPAQEKNFTT APAICHDGKA HFPREGVFVS NGTHWFVTQR NFYEPQIITT DNT FVSGNC DVVIGIVNNT VYDPLQPELD SFKEELDKYF KNHTSPDVDL GDISGINASV VNIQKEIDRL NEVAKNLNES LIDL QELGK YEQGSGYIPE APRDGQAYVR KDGEWVFLST FLSGLEVLFQ GPGGWSHPQF EKGGGSGGGS GGSAWSHPQF EKGGS HHHH HHHH UniProtKB: Spike glycoprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)