[English] 日本語
 Yorodumi
Yorodumi- EMDB-33889: Cryo-EM structure of the DC591053-bound human relaxin family pept... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the DC591053-bound human relaxin family peptide receptor 4 (RXFP4)-Gi complex | ||||||||||||||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||||||||||||||
 Keywords Keywords | human relaxin family peptide receptor 4 / G protein-coupled receptor / ligand recognition / STRUCTURAL PROTEIN | ||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationRelaxin receptors / positive regulation of feeding behavior / negative regulation of adenylate cyclase-activating adrenergic receptor signaling pathway / negative regulation of calcium ion-dependent exocytosis / G protein-coupled adenosine receptor signaling pathway / negative regulation of adenylate cyclase activity / negative regulation of synaptic transmission / positive regulation of neural precursor cell proliferation / G protein-coupled peptide receptor activity / positive regulation of urine volume ...Relaxin receptors / positive regulation of feeding behavior / negative regulation of adenylate cyclase-activating adrenergic receptor signaling pathway / negative regulation of calcium ion-dependent exocytosis / G protein-coupled adenosine receptor signaling pathway / negative regulation of adenylate cyclase activity / negative regulation of synaptic transmission / positive regulation of neural precursor cell proliferation / G protein-coupled peptide receptor activity / positive regulation of urine volume / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / gamma-aminobutyric acid signaling pathway / regulation of calcium ion transport / negative regulation of apoptotic signaling pathway / neuropeptide signaling pathway / neuronal dense core vesicle / positive regulation of vascular associated smooth muscle cell proliferation / positive regulation of superoxide anion generation / Adenylate cyclase inhibitory pathway / response to nutrient / hippocampal mossy fiber to CA3 synapse / Regulation of insulin secretion / G protein-coupled receptor binding / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / cell body / retina development in camera-type eye / GTPase binding / Ca2+ pathway / midbody / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Ras protein signal transduction Similarity search - Function | ||||||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||||||||||||||||||||||||||
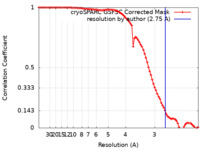
| Method | single particle reconstruction / cryo EM / Resolution: 2.75 Å | ||||||||||||||||||||||||||||||||||||
 Authors Authors | Chen Y / Zhou QT / Wang J / Xu YW / Wang Y / Yan JH / Wang YB / Zhu Q / Zhao FH / Li CH ...Chen Y / Zhou QT / Wang J / Xu YW / Wang Y / Yan JH / Wang YB / Zhu Q / Zhao FH / Li CH / Chen CW / Cai XQ / Bathgate RAD / Shen C / Xu HE / Yang DH / Liu H / Wang MW | ||||||||||||||||||||||||||||||||||||
| Funding support |  China, 11 items China, 11 items
| ||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Ligand recognition mechanism of the human relaxin family peptide receptor 4 (RXFP4). Authors: Yan Chen / Qingtong Zhou / Jiang Wang / Youwei Xu / Yun Wang / Jiahui Yan / Yibing Wang / Qi Zhu / Fenghui Zhao / Chenghao Li / Chuan-Wei Chen / Xiaoqing Cai / Ross A D Bathgate / Chun Shen ...Authors: Yan Chen / Qingtong Zhou / Jiang Wang / Youwei Xu / Yun Wang / Jiahui Yan / Yibing Wang / Qi Zhu / Fenghui Zhao / Chenghao Li / Chuan-Wei Chen / Xiaoqing Cai / Ross A D Bathgate / Chun Shen / H Eric Xu / Dehua Yang / Hong Liu / Ming-Wei Wang /    Abstract: Members of the insulin superfamily regulate pleiotropic biological processes through two types of target-specific but structurally conserved peptides, insulin/insulin-like growth factors and ...Members of the insulin superfamily regulate pleiotropic biological processes through two types of target-specific but structurally conserved peptides, insulin/insulin-like growth factors and relaxin/insulin-like peptides. The latter bind to the human relaxin family peptide receptors (RXFPs). Here, we report three cryo-electron microscopy structures of RXFP4-G protein complexes in the presence of the endogenous ligand insulin-like peptide 5 (INSL5) or one of the two small molecule agonists, compound 4 and DC591053. The B chain of INSL5 adopts a single α-helix that penetrates into the orthosteric pocket, while the A chain sits above the orthosteric pocket, revealing a peptide-binding mode previously unknown. Together with mutagenesis and functional analyses, the key determinants responsible for the peptidomimetic agonism and subtype selectivity were identified. Our findings not only provide insights into ligand recognition and subtype selectivity among class A G protein-coupled receptors, but also expand the knowledge of signaling mechanisms in the insulin superfamily. | ||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33889.map.gz emd_33889.map.gz | 56.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33889-v30.xml emd-33889-v30.xml emd-33889.xml emd-33889.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_33889_fsc.xml emd_33889_fsc.xml | 9.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_33889.png emd_33889.png | 104.5 KB | ||
| Filedesc metadata |  emd-33889.cif.gz emd-33889.cif.gz | 6.9 KB | ||
| Others |  emd_33889_half_map_1.map.gz emd_33889_half_map_1.map.gz emd_33889_half_map_2.map.gz emd_33889_half_map_2.map.gz | 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33889 http://ftp.pdbj.org/pub/emdb/structures/EMD-33889 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33889 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33889 | HTTPS FTP |
-Related structure data
| Related structure data |  7yk7MC  7yj4C  7yk6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33889.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33889.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
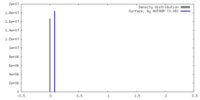
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_33889_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
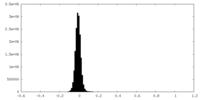
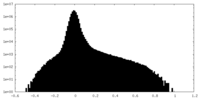
| Density Histograms |
-Half map: #1
| File | emd_33889_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
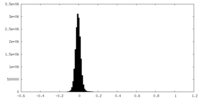
| Density Histograms |
- Sample components
Sample components
+Entire : Cryo-EM structure of the human relaxin family peptide receptor 4 ...
+Supramolecule #1: Cryo-EM structure of the human relaxin family peptide receptor 4 ...
+Supramolecule #2: relaxin family peptide receptor 4
+Supramolecule #3: G protein
+Supramolecule #4: scFv16
+Macromolecule #1: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
+Macromolecule #2: Guanine nucleotide-binding protein G(i) subunit alpha-2
+Macromolecule #3: Relaxin-3 receptor 2
+Macromolecule #4: scFv16
+Macromolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
+Macromolecule #6: [(1S)-7-ethoxy-6-methoxy-1-[2-(5-methoxy-1H-indol-3-yl)ethyl]-3,4...
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source: OTHER |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)