[English] 日本語
 Yorodumi
Yorodumi- EMDB-3365: electron density map of murine leukaemia virus envelope glycoprot... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3365 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | electron density map of murine leukaemia virus envelope glycoprotein as reconstructed by subtomogram averaging on murine leukemia virus virus like particles (3 plasmid system) produced in COS1 cells | |||||||||
 Map data Map data | reconstruction of virus like particle bound murine leukemia virus Env protein | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | murine leukemia virus / retrovirus / envelope glycoprotein / cryo electron tomography / subtomogram averaging | |||||||||
| Biological species |  Murine leukemia virus Murine leukemia virus | |||||||||
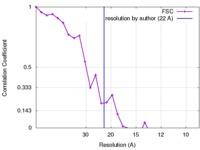
| Method | subtomogram averaging / cryo EM / Resolution: 22.0 Å | |||||||||
 Authors Authors | Riedel C / Vasishtan D / Siebert CA / Whittle C / Lehmann MJ / Mothes W / Grunewald K | |||||||||
 Citation Citation |  Journal: J Struct Biol / Year: 2017 Journal: J Struct Biol / Year: 2017Title: Native structure of a retroviral envelope protein and its conformational change upon interaction with the target cell. Authors: Christiane Riedel / Daven Vasishtan / C Alistair Siebert / Cathy Whittle / Maik J Lehmann / Walther Mothes / Kay Grünewald /     Abstract: Enveloped viruses enter their host cells by membrane fusion. The process of attachment and fusion in retroviruses is mediated by a single viral envelope glycoprotein (Env). Conformational changes of ...Enveloped viruses enter their host cells by membrane fusion. The process of attachment and fusion in retroviruses is mediated by a single viral envelope glycoprotein (Env). Conformational changes of Env in the course of fusion are a focus of intense studies. Here we provide further insight into the changes occurring in retroviral Env during its initial interaction with the cell, employing murine leukemia virus (MLV) as model system. We first determined the structure of both natively membrane anchored MLV Env and MLV Env tagged with YFP in the proline rich region (PRR) by electron cryo tomography (cET) and sub-volume averaging. At a resolution of ∼20Å, native MLV Env presents as a hollow trimer (height ∼85Å, diameter ∼120Å) composed of step-shaped protomers. The major difference to the YFP-tagged protein was in regions outside of the central trimer. Next, we focused on elucidating the changes in MLV Env upon interaction with a host cell. Virus interaction with the plasma membrane occurred over a large surface and Env clustering on the binding site was observed. Sub-volume averaging did yield a low-resolution structure of Env interacting with the cell, which had lost its threefold symmetry and was elongated by ∼35Å in comparison to the unbound protein. This indicates a major rearrangement of Env upon host cell binding. At the site of virus interaction, the otherwise clearly defined bilayer structure of the host cell plasma membrane was much less evident, indicative of integral membrane protein accumulation and/or a change in membrane lipid composition. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3365.map.gz emd_3365.map.gz | 671.7 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3365-v30.xml emd-3365-v30.xml emd-3365.xml emd-3365.xml | 10.1 KB 10.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3365_fsc.xml emd_3365_fsc.xml | 2.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_3365.tif emd_3365.tif | 732.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3365 http://ftp.pdbj.org/pub/emdb/structures/EMD-3365 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3365 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3365 | HTTPS FTP |
-Validation report
| Summary document |  emd_3365_validation.pdf.gz emd_3365_validation.pdf.gz | 264.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_3365_full_validation.pdf.gz emd_3365_full_validation.pdf.gz | 264.1 KB | Display | |
| Data in XML |  emd_3365_validation.xml.gz emd_3365_validation.xml.gz | 6.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3365 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3365 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3365 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3365 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3365.map.gz / Format: CCP4 / Size: 825.2 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3365.map.gz / Format: CCP4 / Size: 825.2 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | reconstruction of virus like particle bound murine leukemia virus Env protein | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.6 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : murine leukemia virus Env protein on murine leukemia virus virus ...
| Entire | Name: murine leukemia virus Env protein on murine leukemia virus virus like particles |
|---|---|
| Components |
|
-Supramolecule #1000: murine leukemia virus Env protein on murine leukemia virus virus ...
| Supramolecule | Name: murine leukemia virus Env protein on murine leukemia virus virus like particles type: sample / ID: 1000 Details: purified virus like particles produced in COS1 cells Oligomeric state: trimer / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 226 KDa |
-Macromolecule #1: murine leukemia virus Env protein
| Macromolecule | Name: murine leukemia virus Env protein / type: protein_or_peptide / ID: 1 / Details: imaged on intact virus like particles / Number of copies: 3 / Oligomeric state: trimer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  Murine leukemia virus / Strain: Friend's murine leukemia virus Murine leukemia virus / Strain: Friend's murine leukemia virus |
| Molecular weight | Theoretical: 226 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Details: DMEM + 10% FSC |
|---|---|
| Grid | Details: C-flat copper grids (Protochips, CF-2/1-2C) |
| Vitrification | Cryogen name: ETHANE-PROPANE MIXTURE / Instrument: OTHER / Method: manually blotted for 3sec |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Specialist optics | Energy filter - Name: Gatan QUANTUM 964 postcolumn energy filter Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Date | Aug 6, 2014 |
| Image recording | Category: CCD / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 60 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 95000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.5 µm |
| Sample stage | Specimen holder model: OTHER / Tilt series - Axis1 - Min angle: -45 ° / Tilt series - Axis1 - Max angle: 45 ° |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Software | Name:  Chimera Chimera |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)