[English] 日本語
 Yorodumi
Yorodumi- EMDB-33590: Cryo-EM structure of the Teriparatide-bound human PTH1R-Gs complex -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Teriparatide-bound human PTH1R-Gs complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Teriparatide / PTH1R / Cryo-EM structure / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmacromolecule biosynthetic process / parathyroid hormone receptor binding / type 1 parathyroid hormone receptor binding / negative regulation of bone mineralization involved in bone maturation / positive regulation of osteoclast proliferation / negative regulation of apoptotic process in bone marrow cell / response to parathyroid hormone / positive regulation of cell proliferation in bone marrow / parathyroid hormone receptor activity / hormone-mediated apoptotic signaling pathway ...macromolecule biosynthetic process / parathyroid hormone receptor binding / type 1 parathyroid hormone receptor binding / negative regulation of bone mineralization involved in bone maturation / positive regulation of osteoclast proliferation / negative regulation of apoptotic process in bone marrow cell / response to parathyroid hormone / positive regulation of cell proliferation in bone marrow / parathyroid hormone receptor activity / hormone-mediated apoptotic signaling pathway / magnesium ion homeostasis / positive regulation of signal transduction / response to fibroblast growth factor / phosphate ion homeostasis / cAMP metabolic process / G-protein activation / Activation of the phototransduction cascade / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Ca2+ pathway / G alpha (z) signalling events / Class B/2 (Secretin family receptors) / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / Adrenaline,noradrenaline inhibits insulin secretion / ADP signalling through P2Y purinoceptor 12 / G alpha (q) signalling events / osteoblast development / response to vitamin D / G alpha (i) signalling events / Thrombin signalling through proteinase activated receptors (PARs) / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / photoreceptor outer segment membrane / G alpha (s) signalling events / positive regulation of inositol phosphate biosynthetic process / G alpha (q) signalling events / spectrin binding / peptide hormone receptor binding / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / bone mineralization / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / alkylglycerophosphoethanolamine phosphodiesterase activity / peptide hormone binding / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / photoreceptor outer segment / chondrocyte differentiation / positive regulation of glycogen biosynthetic process / response to cadmium ion / bone resorption / positive regulation of bone mineralization / Rho protein signal transduction / cell maturation / photoreceptor inner segment / cardiac muscle cell apoptotic process / homeostasis of number of cells within a tissue / skeletal system development / positive regulation of D-glucose import / hormone activity / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / response to lead ion / intracellular calcium ion homeostasis / adenylate cyclase-activating G protein-coupled receptor signaling pathway / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / cell-cell signaling / sensory perception of taste / signaling receptor complex adaptor activity Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Zhai X / Mao C / Shen Q / Zang S / Shen D / Zhang H / Chen Z / Wang G / Zhang C / Zhang Y / Liu Z | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: to be published Journal: to be publishedTitle: Cryo-EM structure of the Teriparatide-bound human PTH1R-Gs complex Authors: Zhai X / Mao C / Shen Q / Zang S / Shen D / Zhang H / Chen Z / Wang G / Zhang C / Zhang Y / Liu Z | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33590.map.gz emd_33590.map.gz | 33 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33590-v30.xml emd-33590-v30.xml emd-33590.xml emd-33590.xml | 29.3 KB 29.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33590.png emd_33590.png | 44.1 KB | ||
| Filedesc metadata |  emd-33590.cif.gz emd-33590.cif.gz | 8.2 KB | ||
| Others |  emd_33590_additional_1.map.gz emd_33590_additional_1.map.gz emd_33590_half_map_1.map.gz emd_33590_half_map_1.map.gz emd_33590_half_map_2.map.gz emd_33590_half_map_2.map.gz | 33 MB 33.1 MB 33.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33590 http://ftp.pdbj.org/pub/emdb/structures/EMD-33590 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33590 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33590 | HTTPS FTP |
-Validation report
| Summary document |  emd_33590_validation.pdf.gz emd_33590_validation.pdf.gz | 811.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33590_full_validation.pdf.gz emd_33590_full_validation.pdf.gz | 810.8 KB | Display | |
| Data in XML |  emd_33590_validation.xml.gz emd_33590_validation.xml.gz | 11.2 KB | Display | |
| Data in CIF |  emd_33590_validation.cif.gz emd_33590_validation.cif.gz | 13.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33590 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33590 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33590 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33590 | HTTPS FTP |
-Related structure data
| Related structure data |  7y36MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33590.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33590.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||||||||||||||||||
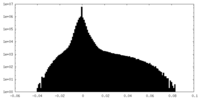
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: half1 map of main map
| File | emd_33590_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half1 map of main map | ||||||||||||
| Projections & Slices |
| ||||||||||||
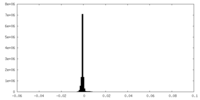
| Density Histograms |
-Half map: half1 map of main map
| File | emd_33590_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half1 map of main map | ||||||||||||
| Projections & Slices |
| ||||||||||||
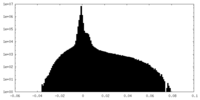
| Density Histograms |
-Half map: half2 map of main map
| File | emd_33590_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half2 map of main map | ||||||||||||
| Projections & Slices |
| ||||||||||||
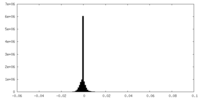
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of the Teriparatide-bound human PTH1R-Gs complex
| Entire | Name: Cryo-EM structure of the Teriparatide-bound human PTH1R-Gs complex |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of the Teriparatide-bound human PTH1R-Gs complex
| Supramolecule | Name: Cryo-EM structure of the Teriparatide-bound human PTH1R-Gs complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Parathyroid hormone/parathyroid hormone-related peptide receptor
| Macromolecule | Name: Parathyroid hormone/parathyroid hormone-related peptide receptor type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 81.730609 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASGK LYPESEEDKE APTGSRYRGR PCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE LVPGHNRTWA NYSECVKFLT NETREREVFD R LAMIYTVG ...String: DADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASGK LYPESEEDKE APTGSRYRGR PCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE LVPGHNRTWA NYSECVKFLT NETREREVFD R LAMIYTVG YSVSLASLTV AVLILAYFRR LHCTRNYIHM HLFLSFMLRA VSIFVKDAVL YSGATLDEAE RLTEEELRAI AQ APPPPAT AAAGYAGCRV AVTFFLYFLA TNYYWILVEG LYLHSLIFMA FFSEKKYLWG FTVFGWGLPA VFVAVWVSVR ATL ANTGCW DLSSGNKKWI IQVPILASIV LNFILFINIV RVLATKLRET NAGRCDTRQQ YRKLLKSTLV LMPLFGVHYI VFMA TPYTE VSGTLWQVQM HYEMLFNSFQ GFFVAIIYCF CNGEVQAEIK KSWSRWTLAL DFKRKARSGS SSYSYGPMVS HTSVT NVGP RVGLGLPLSP RLLPTATTNG HPQLPGHAKP GTPALETLET TPPAMAAPKD DGFLNGSCSG LDEEASGPER PPALLQ EEW ETVMEFVFTL EDFVGDWEQT AAYNLDQVLE QGGVSSLLQN LAVSVTPIQR IVRSGENALK IDIHVIIPYE GLSADQM AQ IEEVFKVVYP VDDHHFKVIL PYGTLVIDGV TPNMLNYFGR PYEGIAVFDG KKITVTGTLW NGNKIIDERL ITPDGSML F RVTINS UniProtKB: Parathyroid hormone/parathyroid hormone-related peptide receptor |
-Macromolecule #2: Parathyroid hormone
| Macromolecule | Name: Parathyroid hormone / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 4.125778 KDa |
| Sequence | String: SVSEIQLMHN LGKHLNSMER VEWLRKKLQD VHNF UniProtKB: Parathyroid hormone |
-Macromolecule #3: Isoform Gnas-2 of Guanine nucleotide-binding protein G(s) subunit...
| Macromolecule | Name: Isoform Gnas-2 of Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 44.257051 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGFNG DSEKATKVQD IKNNLKEAI ETIVAAMSNL VPPVELANPE NQFRVDYILS VMNVPDFDFP PEFYEHAKAL WEDEGVRACY ERSNEYQLID C AQYFLDKI ...String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGFNG DSEKATKVQD IKNNLKEAI ETIVAAMSNL VPPVELANPE NQFRVDYILS VMNVPDFDFP PEFYEHAKAL WEDEGVRACY ERSNEYQLID C AQYFLDKI DVIKQADYVP SDQDLLRCRV LTSGIFETKF QVDKVNFHMF DVGAQRDERR KWIQCFNDVT AIIFVVASSS YN MVIREDN QTNRLQAALK LFDSIWNNKW LRDTSVILFL NKQDLLAEKV LAGKSKIEDY FPEFARYTTP EDATPEPGED PRV TRAKYF IRDEFLRIST ASGDGRHYCY PHFTCAVDTE NIRRVFNDCR DIIQRMHLRQ YELL UniProtKB: Isoform Gnas-2 of Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.44132 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHLEV LFQGPSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHLA KIYAMHWGTD SRLLVSASQ DGKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN LKTREGNVRV SRELAGHTGY L SCCRFLDD ...String: MHHHHHHLEV LFQGPSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHLA KIYAMHWGTD SRLLVSASQ DGKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN LKTREGNVRV SRELAGHTGY L SCCRFLDD NQIVTSSGDT TCALWDIETG QQTTTFTGHT GDVMSLSLAP DTRLFVSGAC DASAKLWDVR EGMCRQTFTG HE SDINAIC FFPNGNAFAT GSDDATCRLF DLRADQELMT YSHDNIICGI TSVSFSKSGR LLLAGYDDFN CNVWDALKAD RAG VLAGHD NRVSCLGVTD DGMAVATGSW DSFLKIWNGS SGGGGSGGGG SSGVSGWRLF KKIS UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.729947 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ASNNTASIAQ ARKLVEQLKM EANIDRIKVS KAAADLMAYC EAHAKEDPLL TPVPASENPF REKKFFCAIL UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #6: NanoBody 35
| Macromolecule | Name: NanoBody 35 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 13.711284 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTV |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Digitization - Frames/image: 1-40 / Number real images: 6947 / Average electron dose: 64.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 49310 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)